Abstract
In this work Cu2ZnSnS4 (CZTS) suitable for the absorption layer in solar cells was successfully prepared by sol–gel spin-coated deposition. CZTS precursors were prepared by using solutions of copper (II) chloride, zinc (II) chloride, tin (IV) chloride, and thiourea. Texture structures with kesterite crystallinity reflected from the X-ray diffraction of (112), (200), and (312) planes of the CZTS were obtained as synthesized at a temperature of over 240 °C. The absorption coefficients of the CZTS films are higher than 104 cm−1, and the optical-energy gap is about 1.5 eV. Without sulfurization treatment, a near stoichiometry composition of the CZTS is obtained at a synthesizing temperature of 280 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
CZTS is a kind of p-type direct band gap semiconductor with a band-gap value of ~1.5 eV. The crystal chemistry of CZTS with kesterite structure has been investigated in recent works [1, 2]. It has the similar stannite structure of CuIn x Ga1−x Se2 (CIGS) and like CIGS is one of the potential materials for solar cells. However, as compared to CIGS, it is of low cost, non-toxic and abundant with a large absorption coefficient in the order of 104 cm−1 [3–5], which make it a suitable absorption layer for applications in thin film solar cells. There are many kinds of methods to prepare CZTS such as spray pyrolysis deposition [6, 7], electron-beam-evaporation [4, 8, 9], rf magnetron sputtering deposition [5, 10], and sol–gel deposition [11]. In this work, the characterization of the CZTS prepared by using sol–gel spin-coated deposition is presented.
2 Experimental methods
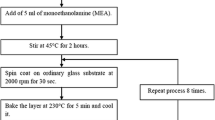
In this work, soda lime glass (SLG) is used as substrates. The SLG substrates were cleaned in an ultrasonic acetone bath for 10 min, and then rinsed with deionized water. CZTS precursors were prepared by using the solutions of copper (II) chloride, zinc (II) chloride, tin (IV) chloride, and thiourea in a mixture of 2, 1, 1, and 8 mol/L, respectively and dissolved in deionized water containing 30 vol% ethanol. The solutions were stirred at 40 °C for 30 min and then spinning coated on the SLG substrate at a speed of 1,350 rpm. After the spin-coated deposition, the films were baked for drying on a hot plate at 110 °C for 10 min, and then synthesized, respectively at a different temperature in a range of 160–320 °C for 20 min. The spin-coated and synthesizing processes were repeated 3 times to obtain a suitable thickness of CZTS.
3 Results and discussion
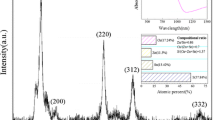
The surface morphology and the cleavage of the CZTS were examined by scanning electron microscope (SEM). Figure 1 shows the cleavage image of the CZTS thin film with a thickness of 2.9 μm, which is obtained as synthesized at 160 °C. As we can see, that the CZTS layer is continuous and uniform. Figure 2 shows the surface morphology of the CZTS synthesized at different temperatures. Texture structures of the CZTS films are formed at a synthesizing temperature of higher than 160 °C. The crystallinity of the CZTS was characterized by an X-ray diffractometer (XRD). From Fig. 3, we can see that the degree of crystallinity of the CZTS increases with synthesizing temperatures. Below 280 °C, the CZTS may not have a complete synthesization, and thus there appears an unidentified phase as shown by the peak locating at near 50°. The CZTS reflects the kesterite structure with the major XRD diffraction from (112), (200), (220) and (312) planes as the synthesizing temperature is higher than 240 °C. This means that in this work the suitable CZTS synthesizing temperature is no less than 280 °C.
The composition ratios of the deposited CZTS thin films were measured by energy dispersive spectroscopy. Figure 4 shows that the composition ratio of sulfur in the CZTS decreases obviously with synthesizing temperatures. The composition ratio of copper is less than 20% as synthesized at 160 °C, and then gradually rises with synthesizing temperatures to a value of close to 25% at 280 °C. Without sulfurization treatment, the CZTS film is found to have a near stoichiometry composition as prepared at a synthesizing temperature of 280 °C.
The transmittance and reflectance of the CZTS films were examined by a photo spectrometer. Figure 5 shows the optical absorption spectra of the CZTS obtained at different synthesized temperatures. The CZTS films have high optical absorption figures with respect to the photo energy of solar irradiation. As we can see, the CZTS films synthesized at a temperature of higher than 160 °C have high optical-absorption properties in a wider band of photon energy. As shown in Fig. 6, the optical-energy gap of the CZTS film is determined by extrapolating the linear part of the spectrum of the square absorption coefficients of the CZTS as a function of photon energy. The average optical-absorption coefficient was derived from the CZTS absorption coefficient with respect to the photo energy from 1.8 to 3.1 eV. As shown in Fig. 7, the average absorption coefficient of the CZTS is higher than 104 cm−1, and the optical energy gap is about 1.5 eV except the sample prepared at 160 °C.
4 Conclusions
In this work, solutions of copper (II) chloride, zinc (II) chloride, tin (IV) chloride, and thiourea are used as the precursors for preparing CZTS thin films. CZTS thin films are successfully prepared on SLG substrates by sol–gel spin-coated deposition. Texture structures of the CZTS films are obtained at a synthesizing temperature of higher than 160 °C. The kesterite structures of the CZTS films result from the synthesizing processes at a temperature of over 240 °C. The CZTS film with a near stoichiometry composition is prepared at a synthesizing temperature of 280 °C. The absorption coefficient and the optical energy gap of the CZTS films are ~2 × 104 cm−1 and ~1.5 eV, respectively.
References
Bernardini GP, Borrini D, Caneschi A, Benedetto FD, Gatteschi D, Ristori S, Romanelli M (2000) Phys Chem Miner 27:453. doi:10.1007/s002690000086
Benedetto FD, Bernardini GP, Borrini D, Lottermoser W, Tippelt G, Amthauer G (2005) Phys Chem Miner 31:683. doi:10.1007/s00269-004-0430-y
Ito K, Nakazawa T (1988) Jpn J Appl Phys 27:2094. doi:10.1143/JJAP.27.2094
Katagiri H, Sasaguchi N, Hando S, Hoshino S, Ohashi J, Yokota T (1997) Sol Energy Mater Sol Cells 49:407. doi:10.1016/S0927-0248(97)00119-0
Seol JS, Lee SY, Lee JC, Nam HD, Kim KH (2003) Sol Energy Mater Sol Cells 75:155. doi:10.1016/S0927-0248(02)00127-7
Kamoun N, Bouzouita H, Rezig B (2007) Thin Solid Films 515:5949. doi:10.1016/j.tsf.2006.12.144
Nakayama N, Ito K (1996) Appl Surf Sci 92:171. doi:10.1016/0169-4332(95)00225-1
Katagiri H (2005) Thin Solid Films 426:480
Katagiri H, Saitoh K, Washio T, Shinohara H, Kurumadani T, Miyajima S (2001) Sol Energy Mater Sol Cells 65:141. doi:10.1016/S0927-0248(00)00088-X
Jimbo K, Kimura R, Kamimura T, Yamada S, Maw WS, Araki H, Oishi K, Katagiri H (2007) Thin Solid Films 515:5997. doi:10.1016/j.tsf.2006.12.103
Tanaka K, Moritake N, Uchiki H (2007) Sol Energy Mater Sol Cells 91:1199. doi:10.1016/j.solmat.2007.04.012
Acknowledgment
This work is supported by the National Science Council of the Republic of China under the contract No. NSC 97-2221-E-022-002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeh, M.Y., Lee, C.C. & Wuu, D.S. Influences of synthesizing temperatures on the properties of Cu2ZnSnS4 prepared by sol–gel spin-coated deposition. J Sol-Gel Sci Technol 52, 65–68 (2009). https://doi.org/10.1007/s10971-009-1997-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-1997-z