Abstract
The effect of an organically modified precursor, 3-glycidoxypropyltrimethoxysilane in an ambient pressure process involving aging in silane solution for silica aerogels is presented. The effect of increasing trialkoxysilane/tetraalkoxysilane precursor ratio and the influence of water to Si molar ratio on the gelation and adsorption properties were investigated. An optimum water to Si molar ratio (8) gave the fastest gelation for all precursor ratios indicating a balance between the increase in rate of hydrolysis and a decrease in concentration of the monomers. Surface area analysis proved that in the dried gel, the organic groups are largely present on the pore walls and prevent the condensation of the silanol groups during drying. This in turn prevents pore collapse and further increases the total pore volume. The inclusion of the organically functionalised silane in the process further enhances the ambient pressure drying through this effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
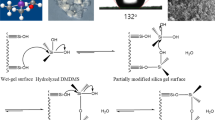
Highly porous silica based materials find applications in a variety of fields and modification of available synthesis routes have not ceased to enthuse researchers [1–3]. Ambient pressure process for aerogel like highly porous silica have attracted considerable interest in recent years owing to the possibility of replacing supercritical drying techniques for such materials [4–7]. Alkylation of the internal surface of the wet gel produces large concentration of alkylgroups on the surface which on close approach produces large repulsive forces. These repulsions result in a spring back effect of the gel network reversing shrinkage during drying. This ingenious approach was first demonstrated by Brinker for the preparation of aerogel films at ambient pressure and later different researchers extended the method to bulk aerogel synthesis [6, 8–10]. Aging the wet gel in a precursor solution results in the strengthening of the gel network and the stiffer network will withstand the drying stress reducing shrinkage when dried at ambient pressure [11–14]. The significance of these techniques is evident from the industrial partnership it has generated [15]. Organically functionalised precursors have played a crucial role in the progress of aerogel technology in recent years. Organic functionalisation has developed from being a method for inducing hydrophobicity in aerogels to a more intricate aspect of nanoengineering mechanically strong aerogels [16]. On the other hand the use of methyltrimethoxysilane precursor has resulted in one of the most fascinating development in the aerogel technology, flexible aerogels [17]. 3-Glycidoxypropyltrimethoxysilane (GPTMS) is another important silane precursor which has applications in a variety of areas like proton conducting membranes, corrosion and scratch resistant coatings, optical applications etc., mainly due to its cross linking capacity through the epoxy group [18–20]. Nevertheless the sol gel chemistry of monofunctional precursors are highly complex and a great deal of investigation has gone into their hydrolysis condensation reactions [21–28]. Trialkoxysilane precursors suffer from the fact that only three alkoxy groups are available for networking, which in turn results in a poorly linked inorganic network upon condensation preventing the formation of monoliths at most conditions [29]. The use of tetraalkoxysilane precursor along with the organically modified silane promotes the inorganic networking facilitating the formation of gels. But due to the difference in rates of hydrolysis and condensation reactions of the two precursors the sol–gel processing of mixed precursors can lead to phase separation and prolonged gelation times. Husing prepared organically modified aerogels with varying organically modified precursors including GPTMS under base catalysed conditions and a water/alkoxide molar ratio of 2 followed by supercritical drying [30]. But ambient pressure drying has not been attempted on such organically modified gels. Further, the hydrolysis, condensation conditions and nature of the catalyst are all variables that can influence the final nature of the gels and the effectiveness of the ambient pressure drying. We have earlier reported on an ambient pressure drying technique on acid catalysed gels involving silane aging and solvent exchange and its successful extension to mixed oxide systems [13, 14, 31]. Here we report on the effect of the GPTMS precursor on the porosity features and adsorption characteristics of acid catalysed gels dried at ambient pressure. The effect of initial water content and the varying precursor ratio are also analysed.
2 Experimental
3-glycidoxypropyltrimethoxysilane and tetraethylorthosilane (TEOS) were procured from Aldrich (Steinheim, Germany) and used as obtained. Isopropyl alcohol and hydrochloric acid were obtained from S.D. Fine chemicals (Mumbai, India). Doubly distilled water was used for the preparation. In a typical preparation weighed amounts of GPTMS and TEOS were mixed with weighed quantity of isopropanol by stirring on a magnetic stirrer. To this solution weighed amount of 10−3N HCl was added. The initial water content is the amount of the acid taken for the preparation of the gel. The solution was stirred for 3 h and poured in to polypropylene vials. The vials were kept at 50 °C for gelation. The gelation times reported are the time at which the flow stops in the vials when tilted [21]. The vials were checked at an interval of 1 h for an initial set and after determining the approximate time, the gelation time was noted for a new set at an interval of 5 min. Gels were prepared with varying GPTMS/TEOS molar ratio and initial water content. Samples with GPTMS/TEOS molar ratio 0.05, 0.1, 0.3 and 0.5 were prepared. The initial water/Si molar ratio was also varied as 4, 8, 12 and 16. Gels are designated as xPyW, where x is the GPTMS/TEOS molar ratio and y is the water/Si molar ratio for the rest of the text. The isopropanol/Si molar ratio was kept constant at 4 for all the preparations. The alcogels were aged in water for a period of 24 h followed by solvent exchange with isopropanol. In order to exchange the solvent, the gels were immersed in isopropanol and the solution replaced with fresh alcohol, 5 times in 24 h. The solvent exchanged gels were then aged in a solution of 80% TEOS in isopropanol for a period of 48 h. The silane aged gels were washed with n-hexane following the same procedure used for solvent exchange. In all the steps the soaking solutions had volume twice as that of the gels immersed in them. The gels were then kept for drying in sealed containers at 70 °C. The container seals were perforated with pins after a day for the solvent to escape slowly. The dried gels were used for further characterization.
Thermal analysis of the gels were performed on a thermogravimetric analyzer (Shimadzu TG 50, Kyoto, Japan) and a differential thermal analyzer (Shimadzu DTA 50, Kyoto, Japan) in air at a heating rate of 5 °C min−1. A fourier transform infrared spectroscope (Magna 560, Nicolet, Madison, Wisconsin) was used for recording the FTIR spectra of the sample. The spectra were acquired using the KBr pellet method in the range 400–4,000 cm−1. Nitrogen adsorption data were obtained using a BET surface area analyzer (Gemini 2360, Micromeritics, Norcross, USA) at 77 K. All analysis were conducted after degassing the sample at 200 °C for 2 h unless specified. The pore size distributions were calculated using the Barrett–Joiner–Halenda method from the desorption curve of the isotherm. All calcinations were performed at a heating rate of 1 °C min−1 and soaked at the respective temperature for 2 h.
3 Results and discussion
The gelation times obtained for the various samples are provided in the Table 1.
The gelation time increases with the GPTMS content. The amount of water used for the hydrolysis also affect the gelation time and there seems to be an optimal amount of water ratio for which the gelation times are low, above and below which gelation time increases. Only the gels prepared at the optimum water ratio was further processed. All the gels cracked after drying. Two gels with internal cracks did not break during measurement and their densities were calculated from mass and dimensions. 0.1P8W had a density 0.22 g cm−3 and 0.3P8W had a density of 0.21 g cm−3. Considering the low values obtained, even with the errors in measurement the densities of these gels should lie in the range for aerogels. Linear shrinkage of the gels were in the range ~24 (0.3P8W)–27% (0.1P8W). Photograph of dried gels are provided as Fig. 1.
The FTIR spectra of all the samples were similar and two are provided as Fig. 2. The peaks were assigned based on available literature [32–34]. The broad absorption between 3,100 and 3,700 cm−1 is due to the OH stretching vibrations. The stretching of alkyl hydrogens are visible around 2,930 cm−1 and the corresponding bending vibrations can be seen around 1,490 cm−1. The absorption around 1,639 cm−1 is due to the bending vibrations of water adsorbed on the silica surface. The Si–O–Si asymmetric stretching vibrations appear as a strong and broad absorption around 1,090 cm−1. The ether linkages in the glycidoxy group also absorb in the same region and will be overlapped by the strong Si–O–Si absorption. The Si–OH asymmetric stretching vibrations can be seen at 950 cm−1. The peak around 800 cm−1 is due to the symmetric Si–O–Si stretching vibrations. The peak at 565 and 460 cm−1 is attributed to the O–Si–O vibrations. The out of plane bending vibrations of C–OH groups can be seen as a weak peak at 698 cm−1.
Thermal analysis of the 0.3P8W gel is provided as Fig. 3. All the prepared gels followed the same pattern. The exothermic peak centred on 230 °C corresponds to the decomposition of the organic part on the silica network. The small endothermic peak observed below 100 °C is due to the removal of water form the gel. The corresponding weight loss can be seen in the TG curve provided in the same figure. The thermogravimetric curve shows a two step weight loss where the first step is due to the removal of adsorbed water. While a total weight loss of ~27% was observed, the weight loss due to removal of water accounts for ~10%. The weight loss becomes constant above 600 °C. Dehydroxylation of surface silanol groups are expected around 600 °C. Assuming that only SiO2 is present above 600 °C and that the decomposition of the glycidoxypropyl groups occur with the cleavage of the Si–C bond and knowing the molar ratio of the precursor, the percentage weight loss due to the decomposition of the glycidoxypropyl group can be calculated. In the case of the 0.3P8W gel the weight loss calculated is around 14%. This is close to the observed weight loss for the second step which indicates that the composition of the gel is well maintained through the preparation. The weight gain during the silane aging step is negligible. The FTIR spectrum of the 0.3P8W after calcination at 600 °C was taken and is compared with that obtained for the 70 °C dried gel in Fig. 4. Upon calcination at 600 °C peaks corresponding to the alkyl hydrogen stretching and bending disappear along with the SiOH stretching at 950 cm−1. The COH absorptions at 698 cm−1 is also removed providing substantial support to the above discussion.
The BET surface area analysis of the gels was conducted to understand the nature of porosity and surface characteristics of the samples. The isotherms obtained for the gels calcined at 250 °C are provided in Fig. 5. The isotherms have the characteristic hysterisis loop of type IV nature. The gels are all mesoporous.
The increase in gelation times with increasing trialkoxy precursor has been observed by many researchers and is attributed to the poor networking ability of the RSiO1.5 network [29]. The rate of hydrolysis of the trialkoxy precursor will be less than the tetraalkoxy precursor due to the steric and inductive effects of the alkyl substitutent. The slower rate of hydrolysis of the trialkoxypercursor ensures that a larger proportion of these molecules are present towards the outer surface of the initially formed clusters as condensation proceeds. The increasing presence of the unhydrolysable glycidoxygroup towards the surface decreases the condensation sites retarding condensation. Husing studied the effect of different alkyl substituted alkoxides in the formation of the gel structure when used as a co-precursor with tetraethoxysilane [30]. For these studies a base catalysed hydrolysis using ammonium hydroxide was followed by supercritical drying of the gels. He did observe the increasing gelation times with the increase in concentration of the alkyl substituted precursor but all compositions were prepared at a water ratio of 1 (OR/H2O). Here we could observe a decrease in gelation time irrespective of the GPTMS content when the water ratio was increased to 8 (H2O/Si). When the water ratio was further raised the gelation time increased. The molar ratio indicates availability of two molecules of water for each alkoxy group when the precursor is tetraalkoxysilane and 2.6 for the trialkoxysilane precursor. The acid catalysed hydrolysis of TEOS is first order with respect to the concentration of water [35]. Hence the rate of hydrolysis reaction of the alkoxide precursor can be considered to increase with the increasing water content. But as the water content increases, alkoxide to alcohol ratio remaining constant, the concentration of the precursor decreases in solution proving detrimental to the condensation reaction rates. The increasing gelation times with increasing water content for the TEOS system is reported [35]. Kretschmer, studied the hydrolysis condensation reactions of GPTMS in a 2 wt% GPTMS aqueous solution (molar ratio ~0.002) at pH 5.4 and found that the molar ratio of condensed species with more than one Si–O–Si linkages approached 50% only after 10 days [24]. In the present study the presence of the catalyst and the higher temperature used for gelation can increase the reaction rates but it is not enough to stifle the influence of the decreasing concentration.
The BET surface area of the gels dried at 70 °C and degassed for 3 h at 150 °C is provided in Table 2. The low surface area values are a clear indication of the presence of alkyl groups on the internal surface of the gels. These groups hinder the adsorption of nitrogen resulting in a decrease in surface area. The decreasing trend in surface area is essentially due to the increase in alkyl groups.
The decomposition of organic groups occur around 240 °C according to the thermal analysis. In order to analyze the surface characteristics after partial removal of the organic groups the samples were calcined at 250 °C for 3 h and then BET analysis was performed. The BET surface area values showed almost a two fold increase with the partial removal of the organic groups. This would mean that the entire network skeleton is made up of the TEOS derived silica particles and the GPTMS molecules condense on to the networked structure. Schubert arrived at similar conclusions for the base catalysed system and we find that similar mechanism acts in the acid catalysed hydrolysis condensation where the network will be more polymeric. The trend for the surface area values is reversed and the gel with the higher content has the larger surface area as expected. There is an increase in pore volume with increasing GPTMS content indicating the effect of GPTMS in the drying stages. The presence of the glycidoxy group on the surface of the pores prevents the condensation of the Si–OH bonds which lead to pore collapse during drying. This effective removal of pore collapse results in the increase in pore volume. The close values of the average pore diameter shows that the pore structure of the gels are similar and that GPTMS has no real influence in the formation of the pore structure. Rather the effect is restricted to the drying stages. The pore size distribution curves obtained for the gels are provided as Fig. 6 and show that similar pore size distribution is present in all the gels.
4 Conclusion
The use of GPTMS as a co-precusor assists the drying at ambient pressure by preventing the condensation of silanols during drying. The gel network is largely dictated by the condensation reactions of TEOS and the influence of GPTMS is observed during the drying stages. The nature of the pores are not affected by the presence of GPTMS but the total pore volume increases with the increasing presence of GPTMS. The use of GPTMS further enhance the ambient pressure drying.
References
J Non-Cryst Solids 285:1–357 (2001)
J Non-Cryst Solids 350:1–404 (2004)
Pierre AC, Pajonk GM (2002) Chem Rev 102:4243–4265
Fidalgo A, Rosa ME, Ilharco LM (2003) Chem Mater 15:2186–2192
Shlyakhtina AV, Oh YJ (2007) In: Proceedings of the 2nd IEEE international conference on nano/micro engineered and molecular systems NEMS '07, pp 365–370
Schwertfeger F, Frank D, Schmidt M (1998) J Non-Cryst Solids 225:24–29
Soleimani Dorcheh A, Abbasi MH (2008) J Mater Process Technol 199:10–26
Prakash SS, Brinker CJ, Hurd AJ et al (1995) Nature 374:439–443
Venkateswara Rao A, Nilsen E, Einarsrud M-A (2001) J Non-Cryst Solids 296:165–171
Hwang S-W, Jung H-H, Hyun S-H et al (2007) J Sol–Gel Sci Technol 41:139–146
Einarsrud M-A (1998) J Non-Cryst Solids 225:1–7
Einarsrud M-A, Nilsen E (1998) J Non-Cryst Solids 226:122–128
Smitha S, Shajesh P, Aravind PR et al (2006) Microporous Mesoporous Mater 91:286–292
Smitha S, Shajesh P, Kumar SR et al (2007) J Porous Mater 14:1–6
Hüsing Nicola, Schubert Ulrich (1998) Angew Chem Int Ed 37:22–45
Leventis N, Sotiriou-Leventis C, Zhang G et al (2002) Nano Lett 2:957–960
Kanamori K, Aizawa M, Nakanishi K, Hanada T (2007) Adv Mater 19:1589–1593
Metroke TL, Kachurina O, Knobbe ET (2002) Prog Org Coat 44:295–305
Tadanaga K, Yoshida H, Matsuda A et al (2003) Chem Mater 15:1910–1912
Innocenzi P, Miorin E, Brusatin G et al (2002) Chem Mater 14:3758–3766
Dong H, Brook MA, Brennan JD (2005) Chem Mater 17:2807–2816
Loy DA, Baugher BM, Baugher CR et al (2000) Chem Mater 12:3624–3632
Dong H, Zhang Z, Lee M-H et al (2007) J Sol–Gel Sci Technol 41:11–17
Buyl F, Kretschmer A (2008) J Adhes 84:125–142
Shen SK, Hu DD (2007) J Phys Chem B 111:7963–7971
Pena-Alonso R, Rubio F, Rubio J et al (2007) J Mater Sci 42:595–603
French SA, Sokol AA, Catlow CRA et al (2006) J Phys Chem B 110:24311–24317
Gigant K, Posset U, Schottner G (2002) Appl Spectrosc 56:762–769
Loy DA, Mather B, Straumanis AR et al (2004) Chem Mater 16:2041–2043
Husing N, Schubert U, Misof K et al (1998) Chem Mater 10:3024–3032
Aravind PR, Mukundan P, Pillai PK et al (2006) Microporous Mesoporous Mater 96:14–20
Jabbour J, Calas S, Gatti S et al (2008) J Non-Cryst Solids 354:651–658
El Rassy H, Pierre AC (2005) J Non-Cryst Solids 351:1603–1610
Yoda S, Ohshima S (1999) J Non-Cryst Solids 248:224–234
Brinker CJ, Scherer GW (1990) Sol–gel science: the physics and chemistry of sol–gel processing. Academic Press, London
Acknowledgments
Authors P. S., S. S., and P. R. A thank CSIR for their fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shajesh, P., Smitha, S., Aravind, P.R. et al. Effect of 3-glycidoxypropyltrimethoxysilane precursor on the properties of ambient pressure dried silica aerogels. J Sol-Gel Sci Technol 50, 353–358 (2009). https://doi.org/10.1007/s10971-009-1926-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-1926-1