Abstract
Silicate produced via the sol–gel process is a biocompatible material that has high purity and high homogeneity. In this study, we evaluated the feasibility of electrospun fibers of silicate formed into silicate nonwoven fabrics (SNF) developed via the sol–gel process as substrates for substance production using Chinese hamster ovarian cells CHO-K1, and as substrates for producing drug metabolism simulators from the human cell line HepG2. We compared the adherent and proliferation profiles of the two cell types on SNF with those profiles produced on a hydroxyapatite-pulp composite fiber sheet (HAPS). During 14 days of cultivation, a greater number of CHO-K1 and HepG2 cells continued to grow on SNF compared to those on HAPS. Per unit volume, the HepG2 cells on SNF showed higher hepatic-specific functions than those on HAPS. These results demonstrate the feasibility of SNF as a cell culture substrate for substrate production, and for producing drug metabolism simulators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electrospinning is a technology for fabricating ultra-fine fibers with diameters from nanometers to micrometers using electrostatic force [1–4]. The main features of nonwoven fabrics composed of ultra-fine fibers include large surface area, high percentage of voids, and small pore size. These features are highly desirable in scaffolds for tissue engineering and in substrates for functional cell cultures such as in three-dimensional model systems [5]. Such scaffolds and substrates have been developed from a variety of materials: collagen, poly-epsilon-caprolactone, poly-lactide-co-glycolide, chitosan, as well as from polyvinyl alcohol and their composites [6–9]. Recently, we reported that silicate fibers prepared by electrospinning technology and via the sol–gel process are useful as scaffolds for bone tissue engineering [10]. Human osteoblastic MG63 cells were found to successfully adhere to individual silicate fibers, and undergo cell proliferation. In addition, the surface of the fibers was covered with particles of apatite after soaking in simulated body fluid for 7 days [10].
Our aim in this study was to evaluate the feasibility of nonwoven fabrics formed from electrospun silicate fibers (SNF) as substrates for the cultivation of mammalian cells for producing substances for biopharmaceutical use and drug metabolism simulators. A variety of reports are available for the application of electrospun fibers toward cell culture substrates. With the exception of our report [10], the common feature associated with the other reports is that the substrates are made from organic polymers [11–14]. Compared with fibers made from organic polymers, inorganic silicate fibers are expected to have a strongly stable composition [15]. In addition, silicate fibers are able to withstand autoclave sterilization. These features are anticipated to be advantageous for developing a culture substrate for use in substance production and for use in producing in vitro drug metabolism simulators.
In this report, we employed SNF over a 2 week period for culturing Chinese hamster ovarian CHO-K1 cells (widely used as transgenic cells for substance production) and the human cell line HepG2 cells (cells typically investigated for use as in vitro metabolic simulators). The growth profiles of both cell types and the hepatic specific function of HepG2 cells on SNF were compared with those grown on a hydroxyapatite-pulp composite sheet (HAPS), a reportedly effective material as a CHO-K1 cell culture substrate [16].
2 Materials and methods
2.1 Preparation of SNF and HAPS
Silica sol was prepared by heating a mixture of tetraethoxysilane, water, ethanol, and hydrogen chloride (molar ratio = 1:2:2:0.01) based on the methods by Choi et al. [4]. Aqueous hydrogen chloride solution was added dropwise into the mixture of ethanol and tetraethoxysilane under stirring using a magnetic stirrer. The resultant mixture was heated at 80 °C for 30 min. The viscosity of the sol gradually increased during the heating period as the sol–gel reaction proceeded. The sol was then cooled at −4 °C to suppress the progress of the reaction. Silica sol was placed in a 10-mL plastic syringe equipped with a 21-gauge stainless steel needle. The tip of the needle was cut at a 90° angle toward the axial direction of the sol flow. The needle connected to the electrode was located 10 cm away from the counterelectrode (tip-to-collector distance). A rotating drum (diameter, 10 cm) covered with aluminum foil and connected to the counterelectrode, was used as the collector. A high-voltage DC generator (Gamma High Voltage Res, FL, USA) was used to apply 10 kV. The electrospun specimens were dried at room temperature overnight to remove the remaining solvents. The specimens were rinsed with distilled water and sterilized using an autoclave before they were used for cell culturing. HAPS used as a control was prepared by a previous method [16]. A hydroxyapatite precursor was prepared from a mixture of calcium nitrate tetrahydrate (Wako, Osaka, Japan), diammonium hydrogen phosphate (Wako, Osaka, Japan), and sodium hydroxide (Wako, Osaka, Japan). One liter of 0.06 mol/L calcium nitrate tetrahydrate and 15 g softwood Kraft pulp were put into a jar fermenter (M-100, Tokyo Rikakikai, Tokyo, Japan). An aqueous solution (300 mL) of 0.12 mol/L diammonium hydrogen phosphate was added dropwise into the jar fermenter and stirred at 50 °C and pH 5. The resultant HAPS was dried at room temperature overnight. The resultant sheet was rinsed with distilled water and sterilized with 70% ethanol before being used for cell culturing.
2.2 Cell cultures
SNF and HAPS were cut into small squares of 1 cm2 and 400-μm thickness, and these specimens were transferred to the individual wells of 24-well tissue culture plates. CHO-K1 cells and HepG2 cells suspended in medium at 5.0 × 104 and 5.0 × 105 cells/mL, respectively, were poured into each well containing these substrates. Dullbecco’s modified Eagle’s medium (Gibco, CA, USA) and Williams’s Medium E (Sigma, MO, USA) supplemented with 10% fetal bovine serum (FBS), 75 μg/mL penicillin, and 50 μg/mL streptomycin were used for cultivation of the CHO-K1 and HepG2 cells, respectively. NH4Cl was added to the medium comprising the HepG2 cells at 1 mM. The cells were cultured in a humidified atmosphere with 5% CO2/95% air at 37 °C. The mediums were exchanged every other day. The number of cells stained with crystal violet was counted using a hemocytometer. The hepatocyte-specific functions of HepG2 cells, albumin secretion and ammonia metabolism functions, were analyzed using a commercial albumin test kit (ALBWELL II, Exocell, PH, USA) and a commercial ammonia test kit (Wako, Osaka, Japan), respectively.
2.3 Micro structural assessment
The morphology of each nonwoven fabric was observed after first coating the fabric with platinum and palladium in an ion coater (E-1030, Hitachi, Tokyo, Japan) using a scanning electron microscope (SEM, S-4500, Hitachi, Tokyo, Japan), and an optical microscope (Nikon DIASHOT, Tokyo, Japan) before and after cell culture. The specimens observed using SEM were treated with 2.5% glutaraldehyde solution (Wako, Osaka, Japan) before drying.
3 Results and discussion
3.1 Cell morphology and growth
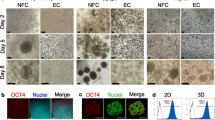
In this study, CHO-K1 and HepG2 cells were seeded on SNF composed of silicate fibers with 300–500 nm diameters (Fig. 1a), and on HAPS composed of apatite and pulp composite fibers with 20–100 μm diameters (Fig. 1b). The vacancy ratios of SNF and HAPS were 93.2% and 77.6%, respectively (Table 1). The CHO-K1 cells seeded onto SNF were found to successfully adhere to the silicate fibers (Fig. 2a) as with those on HAPS (Fig. 2b). The percentage of immobilized CHO-K1 cells on SNF after 1 day of culture was 45%, and there was no significant difference compared with that on HAPS (p = 0.46, Fig. 3a). In the following culture period, CHO-K1 cells continued to grow both on SNF and HAPS. However, the degrees of growth were completely different on each substrate: The cell density of the CHO-K1 cells on SNF after 14 days of culture was 3.6 × 107 cells/cm3 (Fig. 4a), which was three times higher than that on the HAPS substrate. It is obvious that the larger space allowing cellular growth resulting from a higher vacancy ratio in SNF than HAPS is one of the causes of the higher cell density on SNF (Fig. 5a, b). In addition to the difference in the amount of space for cellular growth, a structure composed of ultrafine fibers resulting in less hindrance of oxygen, nutrients and waste exchange between the medium inside and outside of the substrate would be one possible cause of the higher cell density in SNF. The faster growth and higher cell density demonstrates the feasibility of SNF as a substrate for genetically engineered CHO-K1 cell culture toward substance production.
Despite CHO-K1 cells showing almost the same morphologies on SNF and HAPS after 1 day of seeding, the HepG2 cells showed completely different morphologies on each substrate (Fig. 2c, d). The HepG2 cells on HAPS adhered and spread well on fibers (Fig. 2d). In contrast, the cells seeded on SNF produced round shapes between the individual fibers without spreading (Fig. 2c). The result that about 60% of seeded cells remained after rinsing with medium at 1 day of cultivation (Fig. 3b) clearly demonstrates the adherence of HepG2 cells. The number of HepG2 cells on SNF continued to increase over 14 days of cultivation, in a similar manner to that observed with CHO-K1 cells (Fig. 4b). The diameter of the round-shaped cell clusters increased with increasing culture period while the morphology of non-spreading onto the fibers and reached about 100 μm at 14 days of culture (Fig. 6a, b). Such cell clusters were not observed on HAPS (Fig. 6c). Compared with the difference in CHO-K1 cell densities on SNF or HAPS, the HepG2 cell densities were larger at 14 days of cultivation: The HepG2 cell density on SNF was 1.5 × 108 cells/cm3 which is seven times higher than the cell densities of cells cultured on HAPS (Fig. 4b). The enhanced growth of HepG2 cells would also due to the higher vacancy ratio in SNF than HAPS resulting in larger space for cellular growth and less hindrance of oxygen, nutrients and waste exchange between the medium inside and outside of the substrate.
3.2 Liver-specific functions of HepG2 cells
Hepatocytes have many liver-specific functions in the living body. Cell function as well as cell growth is an important indicator in the development of cell culture substrates for use in a drug metabolism simulator [17–20]. In this study, we evaluated two typical hepatocyte-specific functions: the ammonia metabolism rate and the albumin secretion rate. During 14 days of study, both functions of the HepG2 cells cultured on SNF continued to increase (Fig. 7a, b). The degrees of increasing of the hepatocyte-specific functions were greater for SNF than for HAPS: At 14 days of culture, the ammonia metabolism rate and the albumin secretion rate per volume of substrate for SNF were 5 and 10-times faster than those detected for HAPS, respectively. Considering the ammonia metabolism rate of individual cells, SNF had no specific effect on enhancing hepatocyte-specific functions. There was no significant difference between the ammonia metabolism rate of individual cells on SNF and HAPS (p = 0.39, Fig. 8). This result, together with the higher cell density (Fig. 4b); indicate the feasibility of SNF as a substrate for application in drug metabolism simulators with more compact volume.
4 Conclusions
In this study, we evaluated the feasibility of electrospun silicate fibers as a culture substrate for CHO-K1 and HepG2 cells. These cells on SNF grew much faster than those on HAPS. In addition, the hepatocyte-specific functions per volume of substrates for SNF were 5 and 10-times greater than that on HAPS sheet. These results show the feasibility of SNF as functional cell culture substrate for use in substrate production and drug metabolism simulations.
References
Formahals A (1934) US Patent 1975504
Azad M (2006) Mater Lett 60:67
Lyoo S, Youk JH, Lee SW, Park WH (2005) Mater Lett 59:3558
Choi SS, Lee SG, Im SS, Kim SH (2003) J Mater Sci Lett 22:891
Neels JG, Thinnes T, Loskutoff DJ (2004) FASEB J 18:983
Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalto P, Mey J (2007) Biomaterials 28:3012
Li W-J, Danielson KG, Alexander PG, Tuan RS (2003) Biomed Mater Res 67A:1105
Chew SY, Mi R, Hoke A, Leong KW (2007) Biomaterials 29:653
Bin D, Xiaoyan Y, Yuanyuan Z, Xiulan L, Yang Z, Kangde Y (2006) Eur Poly J 42:2013
Sakai S, Yamada Y, Yamaguchi T, Kawakami K (2006) Biotechnol J 1:958
Lannutti J, Reneker D, Ma T, Tomasko D, Farson D (2007) Mat Sci Eng C-Bio S 27:504
Han D (2006) Nanomed Nanotechnol Biol Med 2:37
Kang X, Xie Y, Powell HM, Lee LJ, Belury MA, Lannutti JJ, Kniss DA (2007) Biomaterials 28:450
Kataoka K, Nagao Y, Nukui T, Akiyama I (2005) Biomaterials 26:2509
Ahola MS, Sailynoja ES, Raitavuo MH, Vaahtio MM (2001) Biomaterials 22:2163
Kawakatsu H, Ide S, Okuda Y, Shirahata S (2001) Cytotechnology 35:65
Koide N, Sakaguchi K, Koide Y, Asano K, Kawaguchi M, Matsushima H, Takenami T, Shinji T, Mori M, Tsuji T (1990) Exp Cell Res 186:227
Koide N, Shinji T, Tanabe T, Asano Y, Kawaguchi M, Sakaguchi K, Koide Y, Mori M, Tsuji T (1989) Biochem Biophys Res Commun 161:385
Fukuda J, Sakai Y, Nakazawa K (2006) Biomaterials 27:1061
Fukuda J, Okamura K, Ishihara K, Mizumoto H, Nakazawa K, Ijima H, Kajiwara T, Funatsu K (2006) Cell Transplant 14:819
Acknowledgments
This work was supported by a Grant-in-Aid for Exploratory Research (no. 19760554, 2007) and a Grant-in-Aid for the Global COE Program “Science for Future Molecular Systems” from the Ministry of Education, Culture, Science, Sports and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaguchi, T., Sakai, S. & Kawakami, K. Application of silicate electrospun nanofibers for cell culture. J Sol-Gel Sci Technol 48, 350–355 (2008). https://doi.org/10.1007/s10971-008-1822-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-008-1822-0