Abstract
Effect of amines on an in situ silica generation in natural rubber was investigated, and n-hexylamine, n-heptylamine and n-octylamine were found to increase the in situ silica content. The nanometer sized silica particles up to ca. 80 parts per hundred rubber by weight were generated in situ in the rubber matrix via a sol–gel reaction of tetraethoxysilane. Additionally, dispersion of the silica in the rubbery matrix was more homogeneous than that of commercial silica dispersed by a conventional mechanical mixing. In this in situ silica generation, the polarity and solubility in water of amine were influential factors for controlling the in situ silica content in the rubbery matrix. The obtained high in situ silica filled natural rubber was useful to prepare high performance nanocomposite elastomers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, in situ silica generation has been widely focused as one of the preparation methods for nanocomposites of silica particle and polymers. In the field of soft materials, in situ silica/rubber nanocomposites are expected to afford a high performance elastomer. In addition, the in situ silica/rubber systems are of use in order to clarify a reinforcement effect on rubber, because the nanometer sized silica particles can be dispersed in the rubbery matrix more homogeneously by this method comparing with a conventional mechanical mixing [1, 2]. A pioneered work of the in situ silica generation was carried out by Mark et al. [3], where a sol–gel reaction of tetraethoxysilane (TEOS) was conducted in a crosslinked silicone rubber. In general, the sol–gel reaction of TEOS takes place into two steps, the hydrolysis and the condensation reactions, to generate SiO2 at low temperature [4–6]. The application of in situ silica has been expanded to conventional rubbers since this method was found to be useful for preparing the in situ silica filled styrene-butadiene rubber vulcanizates [7–9]. Crosslinked rubber materials prepared from butadiene rubber [10], epoxidized natural rubber [11] and nitrile rubber [12] also have been subjected to the in situ silica generation. Besides these networks, rubber latexes have also been used for the preparation of rubbery nanocomposites using the sol–gel reaction of TEOS [13, 14]. In addition, several studies have been reported on the chemical modification of rubber molecules by introducing ethoxysilyl groups and the network formation by their sol–gel reaction [15–18].
For further development of this in situ silica generation in the rubber matrix, i.e., in situ silica filling into rubbers by using the sol–gel reaction, the reaction is to be conducted in the uncrosslinked rubbery matrix as well as in the rubber latex. In addition, the reaction conditions for controlling in situ silica content and its size are to be elucidated. The former problem has been solved in our previous studies [19, 20], but the yield of in situ generated silica has not been so high, i.e., less than ca. 45 parts per hundred rubber by weight (phr) [21]. Recently, we have communicated on high silica generation in situ in natural rubber (NR) matrix [22, 23]. In this paper, the details are reported together with the speculated mechanism of generation of in situ silica particles in the TEOS-swollen NR matrix.
2 Experimental
2.1 Materials
NR used was a ribbed smoked sheet (RSS) No.1. TEOS and catalysts (n-butylamine, n-hexylamine, n-heptylamine, n-octylamine, cethylamine, dipropylamine and triethylamine) were purchased from Wako Pure Chemical Industries, Ltd. All reagents for in situ silica generation were reagent grade purity and used as received. The solubility of amines in water [24] is shown in Table 1. The crosslinking reagents for crosslinking were sulfur, N-cyclohexyl-2-benzothiazole sulfonamide (CBS), active zinc oxide and stearic acid. Nipsil VN-3 from Nippon Silica Ind. Co., whose specific surface area was ca. 200 m2/g, was used as commercially available silica for the preparation of reference sample by a conventional milling. Diethylene glycol (DEG) was used for mixing VN-3 with rubber. All reagents for the vulcanization were commercial grade ones.
2.2 Preparation of in situ silica generated NR by sol–gel method
NR sheets of ca. 1 mm thickness were prepared by a two-roll mill. The NR sheets were immersed in TEOS at 40 °C for 1 h and then 25 °C for 16 h. The swollen NR sheets were further immersed in the aqueous solution of catalyst at 40 °C for 72 h in order to conduct the sol–gel reaction of TEOS. The amount of water in the aqueous solution was 5 times of the amount of TEOS obtained in the swollen NR sheets. The products were dried under vacuum at 40 °C until the weight of samples was constant. In order to investigate the effect of catalyst on the sol–gel reaction, amines and their concentration were varied. The conditions of the sol–gel reaction of TEOS in NR are summarized in Table 2. For investigating the effect of reaction time on the in situ silica content, the sheets of 5.5 mm × 2 mm × ca.1 mm were subjected to the sol–gel processing, where the concentration of amines was set 0.096 mol/l.
2.3 Thermogravimetry
In situ silica contents in the products were measured by thermogravimetric analysis (TGA) using a Rigaku Thermal Analyzer Thermoflex TG8110. The sample was placed in a platinum pan and heated up to 1,000 °C under air. The amount of the sample was ca.100 mg and the heating rate was 10 °C/min.
2.4 Transmission electron microscopy (TEM)
Ultra thin films of each specimen were prepared using a MT-XL Ultramicrotome (Boeckeler Instrument, Inc.), and placed on a copper grid which was coated by carbon. Then, the images of TEM were obtained with a transmission electron JEOL TEM-100U instrument. The accelerating voltage was 80 kV.
2.5 Preparation of in situ silica filled nanocomposite elastomer
The in situ silica generated NR of Exp. 6 in Table 2 was mixed with crosslinking reagents by a conventional milling. The recipe is shown in Table 3. All ingredients were mixed on a two-roll mill at r.t. Then, the rubber compound was molded to obtain the vulcanized sheet (NR-71Si) of ca. 1 mm thickness by pressing at 140 °C for 9 min. Before the heat-pressing, a crosslinking reaction of the compound was monitored using a curemeter (Curelastometer III, JSR Co.) at 140 °C in order to determine the adequate crosslinking time. As a reference sample, commercial silica (VN-3) filled NR vulcanizate (NR-71VN) was prepared by a conventional method, whose silica content was 71 phr. In the preparation of NR-71VN, diethylene glycol was added as shown in Table 3 to decrease the negative effect of silica surface on the crosslinking reaction. The heat-pressing was conducted for 16 min at 140 °C. Network-chain density of the samples was determined by the micro-compression method [25, 26] using a Thermo-Mechanical Analyzer (TMA60 of Shimadzu Co.). The small specimen with a size of 2 mm × 2 mm × ca.1 mm was soaked in toluene for 24 h at 25 °C. Then, the swollen sample was compressed by loading at 100 g/min.
2.6 Tensile measurement
Tensile properties of the vulcanized samples were performed with a tensile tester (Autograph, Shimadzu Co.). The measurements were carried out by using tensile speed of 20 mm/min at r.t. Ring-shaped samples were subjected to the measurement, whose outside and inside diameters were 13.7 and 11.7 mm, respectively.
3 Results and discussion
3.1 Effect of amines on in situ silica generation in NR matrix
In order to increase the amount of in situ silica in the rubbery matrix, we have studied a few reaction conditions of the sol–gel process, and the amount of generated in situ silica was found to significantly depend on the amount of TEOS in the swollen samples. The maximum of in situ silica content, however, was ca.45 phr, when n-butylamine was used as a catalyst in the sol-gel reaction of TEOS in NR matrix [21]. Thus, effect of amines was investigated for increasing the amount of in situ silica in this study. For the quantitative discussion, the swelling degree of NR in TEOS was set in the range of 344–367% as shown in Table 2. In searching a good catalyst for a higher in situ silica generation, two points are considered; one is polarity and the other is basicity of the catalyst. The former may be important to transport the catalyst smoothly into the TEOS-swollen matrix from the aqueous solution of catalyst. It is because the reaction system in this study is composed of two phases, i.e., a hydrophobic part of TEOS-swollen NR matrix and a hydrophilic part of aqueous solution of catalyst. The basic catalyst is preferable to promote the three dimensional growth of Si–O–Si linkage [6]. Thus, the effect of methylene group of primary alkylamines was investigated in this Section.
Effect of primary amines on the yield of generated in situ silica is shown in Table 2, where n-butylamine, n-hexylamine and n-octylamine are compared. It is noted that increase of the methylene unit in the primary amine from 4 (n-butylamine) up to 8 (n-octylamine) gave a significant increase of in situ silica content in the uncosslinked NR matrix at the catalyst concentration of 0.096 mol/l. The long hydrocarbon chain of primary amine promoted the in situ silica generation in NR matrix. The difference between n-hexylamine and n-octylamine was not much significant.
Effect of type of amine on the sol–gel reaction of TEOS is also investigated, where primary, secondary and tertiary amines of the same molar mass (101.19 g/mol) are compared: They are n-hexylamine, dipropylamine and triethylamine, and the experimental conditions are shown in Exps. 2, 4 and 5 of Table 2. It is clearly observed that the highest amount of in situ silica in the uncrosslinked NR matrix was obtained by using n-hexylamine, and the silica content significantly decreased by using dipropylamine and triethylamine. The in situ silica content by secondary amine was one-half, and that by tertiary amine was one-sixth compared with that of primary amine. These results suggest that the polarity and the steric factor of amine molecules remarkably influence the sol-gel reaction of TEOS in NR matrix under the experimental conditions of this study. The polarity affects the penetration of amine into the TEOS-swollen NR, because the matrix was hydrophobic. The lower the polarity of the catalyst was, the larger the amount of catalyst that has migrated into the rubbery matrix. Generally, the polarity of amine decreases with the increase of length of hydrocarbon chain in primary amines and it decreases as follow; primary amine > secondary amine > tertiary amine [27]. In secondary and tertiary amines, not only polarity but also steric hindrance may contribute to the lower transportation to the rubbery matrix. Thus, the low polarity accelerated the migration of amine into the TEOS-swollen NR matrix from the aqueous solution of catalyst, which must have promoted the sol–gel reaction of TEOS to generate in situ silica of high content. The details on the effect of steric factor will be discussed with the results of TEM observation in the Sect. 3.4.
Here, we also consider difference of solubility of amines in water, because amines were dissolved in water before the sol–gel reaction of TEOS. The solubility in water of amines used in this study is summarized in Table 1. The solubility in water decreases with the increase of methylene group of the primary amines and it decreases as follows; primary amine > secondary amine > tertiary amine. These orders are in good agreement with the amount of in situ silica content in the products, i.e., the smaller the solubility in water was, the larger the amount of in situ silica among the amines shown in Table 2. However, it is noted that the amount of in situ silica generation decreased when cethylamine (CH3(CH2)15NH2) was used as a catalyst for the sol–gel process, although its polarity is lower than that of n-octylamine. Most of cethylamine floated on the aqueous solution in the experiment, and in situ silica particles were mainly generated on the surface of NR sample. Namely, these results showed that the homogeneous generation of in situ silica in NR matrix did not occur when cethylamine was used. Thus, the results by cethylamine are not included in Table 2. This observation clearly suggests adequate solubility of primary amine in water is also important for the high in situ silica generation in the rubbery matrix.
3.2 Effect of reaction time on in situ silica generation in NR matrix
Effect of reaction time on the amount of generated in situ silica was investigated, where small rubber sheets were taken from the reaction vessel at each reaction time. In Fig. 1, the relationship between the reaction time and the amount of in situ generated silica is displayed for the systems using n-hexylamine, n-heptylamine and n-octylamine. It is worth noting that the maximum in situ silica content was attained within ca.10 h in all systems. It is found that the use of these primary alkylamines results in not only the increase of in situ silica content but also the acceleration of the sol–gel reaction, which are preferable for the practical application of this method into rubber.
3.3 Effect of n-hexylamine concentration on in situ silica generation in NR matrix
Effect of the n-hexylamine concentration on the conversion of TEOS and the silica content is shown in Table 2 (Exp. 2, 6–9) and Fig. 2. Since the solubility in water of n-hexylamine is higher than those of n-heptylamine and n-octylamine, n-hexylamine afforded a more homogeneous reaction system. Thus, n-hexylamine was further subjected to the investigation. The amount of generated in situ silica increased with the increase of n-hexylamine concentration. The yield increased significantly at the lower concentration of the catalyst, but it seemed to attain an asymptotic value of ca.80 phr at the high concentration as shown in Fig. 2. This result suggests that the rate of sol–gel reaction increased with increasing the concentration of n-hexylamine. However, the reaction may have reached equilibrium when the catalyst concentration was sufficient for the reaction of TEOS under the present reaction conditions. It is also observed that the aqueous solution was clear throughout the sol–gel reaction, when the concentration of n-hexylamine was set at less than 0.096 mol/l. TEOS in the rubbery matrix was suggested to migrate to the aqueous solution of catalyst when its concentration was higher than a certain value. Thus, 0.064 mol/l aqueous solution of n-hexylamine is concluded to be the most adequate for the effective generation of in situ silica in the rubbery matrix.
3.4 Morphologies of in situ silica in NR as revealed by TEM
For the in situ silica generation in rubbery matrix, a study on morphology of the in situ silica particles in the matrix is important, because not only the content and the particle size but also the dispersion of silica particles significantly influences the properties of the nanocomposite elastomers. Figure 3a–d shows the TEM photographs of in situ silica particles generated in the uncrosslinked NR matrix by using n-hexylamine with various concentrations. Figure 3e–h show the particles generated by n-octylamine, n-butylamine, dipropylamine and triethylamine, respectively, at the same concentration of 0.096 mol/l. The particle size of in situ silica for all samples is summarized in Table 2, where the accuracy on the measurement was governed by the experimental error. All in situ silica particles were generated in the level of nanometer size and they were in the spherical shape with the relatively homogeneous particle size except in the case of triethylamine. From Fig. 3a–d, it is obvious that the particle size of in situ silica tended to increase with the increase of concentration of n-hexylamine, which was in good agreement with those of the previous studies using the different catalysts [21, 28]. Comparing with the dispersion of silica particles by mechanically mixing, fairly homogeneous dispersion of in situ silica particles in NR matrix was also observed in their TEM photographs. The comparison of TEM images by using various kinds of catalysts at the same concentration of 0.096 mol/l indicates that the reaction by n-hexylamine and n-octylamine gave more homogeneous dispersion of silica than that of n-butylamine. The silica aggregates became larger by using dipropylamine and triethylamine. Especially, in the case of triethylamine, the various sizes of in situ silica particle were generated and fused further into aggregates as shown in Fig. 3h.
TEM images of in situ silica in uncured NR matrix (a) n-hexylamine 0.008 mol/l, (b) n-hexylamine 0.016 mol/l, (c) n-hexylamine 0.064 mol/l, (d) n-hexylamine 0.096 mol/l, (e) n-octylamine 0.096 mol/l, (f) n-butylamine 0.096 mol/l, (g) dipropylamine 0.096 mol/l, (h) triethylamine 0.096 mol/l. The horizontal bar corresponds to 100 nm
3.5 Mechanism of formation of in situ silica particles by the sol–gel reaction in rubbery matrix
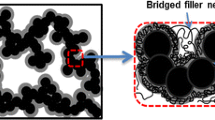
The mechanism of generation of in situ silica particles of high content in NR matrix is proposed as follows. The primary alkylamines with long hydrocarbon segments are estimated to form reverse micelles like a surfactant in the TEOS-swollen NR matrix [29, 30] as shown in Fig. 4 on the basis of observation by TEM photographs. Inside of the reverse micelles, water seems to be capsulated. In the interface between the reverse micelles and the aqueous solution of catalyst, the hydrolysis reaction of TEOS is speculated to occur followed by the generation of hydrophilic products with silanol groups. The products may migrate into the reverse micelles, and the condensation reaction of the silanol groups may progress to generate spherical silica in the reverse micelles. The reverse micelles formed by primary alkylamine with a long hydrocarbon segment may have a repulsive interaction with each other to result in homogeneous dispersion of silica particles in the TEOS-swollen rubber matrix. The stability of reverse micelles must be higher in alkylamines with longer primary chain and less branched structure among the catalysts in Table 2. These factors are speculated to bring about the more homogeneous spherical and nanometer sized silica particles in the NR matrix. The in situ silica content must also be influenced by the stability of reverse micelles. In addition, a supply of water into the TEOS-swollen NR matrix is important in order to increase the in situ silica content, because water is necessary to progress the sol–gel reaction of TEOS. The reverse micelles seem to play a role to transport water when they migrate from the aqueous solution of catalyst into the TEOS-swollen NR matrix.
Generally, the size of reverse micelles was reported to be dependent on the molar ratio between a surfactant and water, i.e., the higher the molar ratio of surfactant, the smaller the size of reverse micelles [31]. In this study, however, both size and content of the in situ silica particles were increased with the increase of concentration of catalyst, when molar ratio of n-hexylamine in water was valid as shown in Table 2. Since alkylamines were used in this study, the amino group catalyzed to promote the sol–gel reaction of TEOS to generate in situ silica particles. Thus, the larger silica content and size were achieved by increasing the amount of n-hexylamine in the range of catalyst concentration studied here.
3.6 Tensile properties of in situ silica filled NR nanocomposites
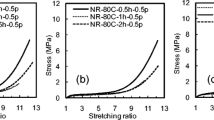
Tensile stress-strain curve of in situ silica filled nanocomposite elastomer (NR-71Si) prepared from the sample (Exp. 6 in Table 2) is shown in Fig. 5. As a reference, tensile stress-strain curve of commercial silica VN-3 filled NR vulcanizate (NR-71VN) is displayed in this figure. It is clear that in situ silica filled sample showed the lower stress at the elongation up to ca. 200% and higher stress at the elongation beyond ca.200% even thought the network-chain densities of these samples were comparable (7.60 × 10−5 mol/cm3 for NR-71Si and 7.21 × 10−5 mol/cm3 for NR-71VN). The former observation suggests the better dispersion of in situ silica particles than that of VN-3 silica in the NR matrix, which was confirmed by TEM as shown in Fig. 6. In fact, the high modulus at low elongation for conventional silica filled rubber has been assumed to be due to the formation of larger aggregates of silica. The latter observation is ascribable to the stronger interaction between in situ silica particle and NR molecules to increase the tensile stress. The high in situ silica generation gave an excellent reinforcement effect on NR vulcanizate. Not only the direct use but also the diluted use of the obtained high in situ silica filled NR was available to prepare the various kinds of NR nanocomposite elastomers. The detail will be reported elsewhere in near future.
4 Conclusions
The effects of amine catalyst and its reaction condition on the sol-gel reaction of TEOS in the uncrosslinked NR matrix were investigated in detail aiming to obtain a high in situ silica generation in NR. The polarity of amine was important for controlling the in situ silica generation in rubbery matrix. The primary alkylamines with pertinent hydrocarbon segments, i.e., n-hexylamine, n-heptylamine and n-octylamine, gave the high contents up to ca.80 phr of homogeneous in situ silica particles in NR matrix with fairly homogeneous dispersion within the reaction time of ca.10 h. The amount of in situ silica increased with the increase of concentration of n-hexylamine. Due to the higher solubility in water, n-hexylamine was found to be the most preferable catalyst for the effective in situ silica generation. The high performance nanocomposite elastomer was prepared from the high in situ silica filled NR even by using a sulfur crosslinking system without diethylene glycol. This method will be useful as a new technology for development of soft matters.
References
Erman B, Mark JE (1997) Structures and properties of rubberlike networks. Oxford University Press, New York
Kohjiya S, Ikeda Y (2000) Rubber Chem Technol 73:534
Mark JE, Pan S-J (1982) Macromol Chem Rapid Commun 3:681
Brinker CJ, Scherer GW (1990) Sol-gel science: the physics and chemistry of sol-gel processing. Academic Press, New York
Hench LL, Ulrich RD (1985) Science of ceramic chemical processing. John Wiley & Sons, New York
Sakka S (1988) The science of the sol-gel process. Agune Shofusya, Tokyo (in Japanese)
Kohjiya S, Yajima A, Yoon JR, Ikeda Y (1994) Nippon Gomu Kyokaishi 67:859 (in Japanese)
Ikeda Y, Tanaka A, Kohjiya S (1997) J Mater Chem 7:445
Hashim AS, Azahari B, Ikeda Y, Kohjiya S (1998) Rubber Chem Technol 71:289
Ikeda Y, Kohjiya S (1997) Polymer 38:4417
Hashim S, Ikeda Y, Kohjiya S (1995) Polym Int 38:111
Tanahashi H, Osanai S, Shigekuni M, Lio S, Murakami K, Ikeda Y, Kohjiya S (1998) Rubber Chem Technol 71:38
Toutorski IA, Tkachenko TE, Maliavski NI (1998) J Sol-Gel Sci Technol 13:1057
Yoshikai K, Ohsaki T, Furukawa M (2002) J Appl Polym Sci 85:2053
Yamashita S, Yamada A, Ohata M, Kohjiya S (1985) Makromol Chem 186:1373, 2269
Hashim AS, Ikeda Y, Kohjiya S (1995) Polym Int 38:111
Gonzalez L, Rodríguez A, de Benito JL, Marcos-Fernández A (1997) J Appl Polym Sci 63:1353
Sunada K, Takeshita H, Miya M, Nakamura T, Takenaka K, Shiomi T (2003) Nippon Gomu Kyokaishi 76:234
Kohjiya S, Murakami K, Iio S, Tanahashi T, Ikeda Y (2001) Rubber Chem Technol 74:16
Murakami K, Lio S, Ikeda Y, Ito H, Tosaka M, Kohjiya S (2003) J Mater Sci 38:1447
Ikeda Y, Kameda Y (2004) J Sol-Gel Sci Technol 31:137
Poompradub S, Kohjiya S, Ikeda Y (2005) Chem Lett 43:672
Ikeda Y, Poompradub S (2006) PCT/Japanese Patent 2006/306124
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, Ithaca, NY
Nakauchi H, Utsunomiya T, Masuda K, Inoue S, Naito K (1987) Nippon Gomu Kyokaishi 60:267
Solomons TWG, Fryhle CB (2004) Organic chemistry, 8th edn. John Wiley & Sons, USA
Breiner JM, Mark JE, Beaucage G (1999) J Polym Sci Part B Polym Phys 37:1421
Osseo-Asare K, Arriagada FJ (1999) J Colloid Interface Sci 218:68
Debuigne F, Jeunieau L, Wiame M, Nagy JB (2000) Langmuir 16:7605
Nippon Yushi Gakkai (ed) (2005) Kaimen to Kaimen Kasseizai, Tokyo, p.201 (in Japanese)
Acknowledgement
This research was partially supported by Grant-in-Aid for Science Research (C) No. 19550208 from JSPS and the Research Grants from Hosokawa Powder Technology Foundation (2005) and President of KIT to Y. I.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikeda, Y., Poompradub, S., Morita, Y. et al. Preparation of high performance nanocomposite elastomer: effect of reaction conditions on in situ silica generation of high content in natural rubber. J Sol-Gel Sci Technol 45, 299–306 (2008). https://doi.org/10.1007/s10971-008-1682-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-008-1682-7