Abstract
Platinum catalysts supported on indium-doped alumina were prepared by the sol–gel method. The method allows the incorporation of In3+ in the alumina network. The indium-doped alumina supports showed narrow pore size distribution (5.4–4.0 nm) and high specific surface areas (258–280 m2/g). The 27Al NMR-MAS spectroscopy identified aluminum in tetrahedral, pentahedral, and octahedral coordination; however, the intensity of the signal assigned to aluminum in pentahedral coordination diminishes with the increase of the content of indium. Total acidity determined by ammonia thermodesorption diminishes strongly in Pt/In–Al2O3 catalysts, suggesting a selective deposit of platinum over the acid sites of the support. The effect of the support in the platinum catalytic activity was evaluated in the n-heptane dehydrocyclization reaction. The selectivity patterns for such reaction were modified substantially in the doped Pt/In–Al2O3 catalysts, in comparison with the Pt-In/Al2O3–I coimpregnated reference catalyst. As an important result, the formation of benzene was suppressed totally over the indium-doped alumina sol–gel supports with a high content (3 wt%) of indium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 1 Introduction
The new trends in reformulation of fuel show an important reduction in the level of aromatics allowed in the pool of gasolines. Therefore, an important unresolved problem is the high content of aromatics in the gasoline produced by the naphtha reforming units, which use Pt–Ir, Pt–Re, or Pt–Sn alumina-supported catalysts [1–4]. These aromatics, especially benzene, which is reported as a carcinogenic active molecule, must be diminished from the gasoline pool at levels lower than 1% vol. New formulations of bimetallic or trimetallic reforming platinum catalysts containing Ge [5, 6], Ga [7, 8], or In [9–12] have been reported; however, the yield in aromatic compounds obtained with them continues to be important.
The formation of aromatic compounds in reforming units is carried out by means of a reaction that involves the bifunctional properties of the catalysts: the metallic phase and the support acidity. Hence, the development of improved new formulations must also be directed to the modifications of the support properties. In this way, it has been reported that the doping of alumina gel [13–16] or Boehmite [17, 18] with tin chloride diminishes the formation of benzene notably in the hydrocarbons dehydrocyclization over Pt–Sn/Al2O3 bimetallic catalysts. In such preparations, the effect of tin in the performance of the bimetallic catalysts was mainly located in the textural and structural modifications of the support rather than in the bimetallic Pt–Sn phase.
In the present paper, we report the preparation of platinum reforming catalysts using indium-doped alumina as support, in order to get a low yield in aromatic compounds in the reforming units. Indium was chosen as the doping precursor, since it has recently been reported as a good performer for the transformation of hydrocarbons over bimetallic Pt–In alloys supported in alumina [9–12]. However, in our catalysts synthesis, we modified at the same time the properties of both: the support and the metallic phase. First, the textural and structural properties of the alumina support were modified by adding indium acetylacetonate to the alumina gel. The alumina structure was perturbed and modification in the acidity was obtained. Second, the platinum activity was modified by its interaction with the highly dispersed In2O3 particles.
The characterization of the catalysts was made by means of nitrogen adsorption isotherms, XRD spectroscopy, ammonia-TPD, and 27Al NMR-MAS. The catalytic activity was evaluated in the n-heptane conversion at 490 °C. Moreover, in order to make a comparison of the behavior of our catalysts in this reaction, the evaluation of Pt–In/Al2O3 bimetallic catalysts, prepared by impregnation of the alumina bare support is also reported.
2 2 Experimental
2.1 2.1.1 sol–gel catalysts preparation
Al2O3 and In–Al2O3 supports were prepared by the sol–gel method as follows: to a solution containing 238 mL of ethanol (J. T. Baker 96%) and 14 mL of distilled water the pH was adjusted to 5 using glacial CH3COOH acid (J. T. Baker 99.7%). After that, it was simultaneously added to the 70 mL solution (3:1 vol. ethanol–water) containing the appropriate amount of indium acetylacetonate (Chemat 98%) and 48 g of aluminum tri-sec-butoxide (Aldrich to 97%). Then, the solution was maintained under reflux and stirred for 72 h at 70 °C. The concentration of indium in the supports was calculated in order to obtain 1 and 3 wt%, respectively. Once the gel was formed, it was dried in a rotavapor at 80 °C. Then, the sample was dried in an oven at 80 °C for 12 h and annealed at 600 °C for 4 h, with a heating program rate of 2 °C/min. The alumina support was prepared with the same technique as the one used for indium–alumina supports, but this time without indium acetylacetonate in the ethanol–water solution. The annealed supports were labeled as InX–Al2O3, where X denotes the indium content.
Platinum sol–gel catalysts: Pt/Al2O3 and Pt/In–Al2O3 were prepared by incipient wetness impregnation of the supports with the appropriated quantity of a solution containing platinum acetylacetonate in acetone, in order to obtain 0.5 Pt wt%. The excess of water was evaporated in a rotavapor and the solid was dried at 80 °C for 12 h and then calcined in air at 600 °C for 4 h. Finally, the catalysts were reduced in hydrogen flow (1.8 L/h) at 500 °C for 2 h.
2.2 2.1.2 Coimpregnated catalyst preparation
Pt–In/Al2O3–I catalysts were prepared by impregnation of the alumina sol–gel support stabilized at 600 °C with an acetone solution containing indium acetylacetonate in the appropriate amount to obtain 1 wt% In. After that, the excess of acetone was evaporated in a rotavapor and then calcined at 600 °C in air. The platinum was incorporated by a subsequent impregnation of the calcined In/Al2O3 support with an acetone solution containing platinum acetylacetonate in the appropriate amount to obtain 0.5 wt% of Pt. Finally, the catalyst was calcined at 600 °C and then reduced at 500 °C in hydrogen flow for 2 h.
2.3 2.2 Characterization
Specific surface areas of the samples annealed at 600 °C were determined with a Quantachrome Multistation Autosorb-3B apparatus, using nitrogen as adsorbate. All the catalysts were degassed under vacuum at 350 °C for 12 h before each measurement. The specific surface area was evaluated by the BET method and the pore size diameter was calculated by the BJH method. The accuracy of the calculation methods and apparatus is ±5%.
XRD spectra patterns were recorded on a Siemens D500 diffractometer using CuKα radiation.
Nuclear magnetic resonance-magic angle spinning (NMR-MAS) 27Al spectra were recorded with an ASX300 spectrometer (Bruker) at 10 KHz under room conditions.
Ammonia thermodesorption was carried out using a TPR-TPD Micromeritics equipment Mod. 2900. First, the samples were treated at 350 °C for 1 h in He flow. Then, the He flow was switched at room temperature by a gas mixture (80/20 vol%) of He/NH3 for 20 min. Afterward, ammonia was desorbed in He flow (30 mL/min) with a program rate of 10 °C/min to reach up to 600 °C. The determination in various samples gave an accuracy of ±5%.
Platinum metal dispersion was determined by CO-pulse chemisorption with a TPD/TPR 2900 Micromeritics apparatus. Before CO chemisorption, the samples were treated at 600 °C for 1 h in air flow. Then, the temperature was put down to room temperature and a H2 flow was switched. After that, the temperature was raised to 500 °C and maintained for 1 h in H2 flow, the hydrogen was purged at 50 °C with He flow, and then pure CO was admitted in the cell by pulses until saturation. Platinum dispersion was calculated assuming a stoichiometric factor CO/Pt = 1. The reproducibility of the values was in the range of ±10%.
2.4 2.3 Catalytic test
The n-heptane conversion was evaluated in a Pyrex U-tube flow reactor (3 mL) at atmospheric pressure. Catalyst samples (100 mg) were pretreated with H2 at 500 °C for 1 h, using a flow rate of 3.6 L/h. The n-heptane was passed through the reactor using a saturator and hydrogen as carrier gas. The partial pressure for n-heptane was 11 Torr, the H2 feed flow 3.6 L/h, and the reaction temperature was 490 °C.
The effluent gas mixture was analyzed with an online gas chromatograph (Varian GC-3800) equipped with a capillary column PE1-PONA (30 × 0.32 mm and 0.25 μm film thickness) and flame ionization detector (FID). Each sample was evaluated three times and an average of the values was taken. They fluctuate in ±10% for conversion and ±5% in selectivity.
3 3 Results and discussion
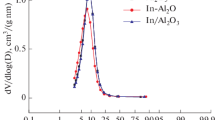
The specific surface area, pore volume, and mean pore size diameter obtained for the various sol–gel supports and sol–gel catalysts are reported in Table 1. For the supports, the addition of indium acetylacetonate to the alumina gel slightly decreases the specific surface areas from 303 m2/g for the alumina to 258 m2/g and 280 m2/g for the In–Al2O3 sol–gel supports at 1 and 3 In wt%, respectively. A similar effect in the specific surface area has also been reported on Sn-Al2O3 sol–gel prepared supports [19]. On the other hand, smaller pore size diameter was obtained in the In–Al2O3 doped supports than on the alumina reference support. For the alumina, the pore size was 13.4 nm, whereas for In–Al2O3 supports were 5.5–6.0 nm. In Fig. 1, a very narrow pore size distribution can be seen for In–Al2O3 supports, while for Al2O3 reference a broad curve was observed, suggesting important structural rearrangements on the indium-doped alumina supports. Additional effects in the textural properties were not observed after impregnation with platinum.
For the Pt–In/Al2O3–I bimetallic catalyst prepared by coimpregnation, the specific surface area and the mean pore size diameter are of the same order as that obtained on the alumina reference sample (Table 1). Additionally, the pore size distribution was not modified substantially, showing a broad curve similar to that obtained on the bar alumina sol–gel support; i.e., the effects in the pore size distribution were only obtained when In was added as a dopant to the alumina gel.
Platinum dispersion was determined by CO chemisorption and the results are reported in Table 1. Values between 33 and 38%D were obtained for sol–gel preparations, whereas for the coimpregnated catalyst, the platinum, the dispersion was 66%D. The higher dispersion obtained in the coimpregnated catalyst was also observed in similar preparations Pt + (In/Al2O3) reported elsewhere [11], where the highest H/Pt = 0.63 ratio was obtained in comparison to In + (Pt/Al2O3).
The XRD profiles for the In–Al2O3 doped supports annealed at 600 °C are presented in Fig. 2. All the supports showed the γ-alumina phase but their profiles are not well resolved since we have alumina with high specific surface area. It is noticeable that indium or indium oxide characteristic peaks are not observed in these samples. However, in samples sintered at 1200 °C, the XRD spectra showed clearly the peaks assigned to In2O3 and Pt3O4 (Fig. 3). Obviously, at such annealing temperature, alpha alumina was formed. The absence of the characteristic In2O3 peaks in samples annealed at 600 °C can be due to the formation of highly dispersed indium oxide, whose particles cannot be resolved by XRD spectroscopy. For the coimpregnated Pt–In/Al2O3–I catalyst, the XRD diffraction patterns (not shown) are similar to that observed in sol–gel preparations.
27Al NMR-MAS spectra were obtained for the various supports (Fig. 4). Three peaks centered at 55–60, 28–34, and 2–3.5 ppm assigned to aluminum in tetrahedral, pentahedral (or deformed tetrahedral), and octahedral coordination, respectively, can be observed [20, 21]. The aluminum in pentahedral coordination appears with the major relative abundance in Al2O3 and In–Al2O3–I supports. However, it practically disappears in the In–Al2O3 sol–gel doped samples. We assume that indium (In3+) perturbs the formation of pentahedral coordinated aluminum. For the Pt/Al2O3 and for the coimpregnated Pt–In/Al2O3–I catalysts, the pentacoordinated aluminum was always present. It does not disappear by indium effect as for such catalysts indium was added by impregnation of the alumina sol–gel support; i.e., indium was not inserted into the alumina network and no modifications on the relative abundance of aluminum in tetrahedral, pentahedral, or octahedral coordination were provoked.
The acidity of the doped sol–gel catalysts was determined by NH3-TPD and the results are reported in Table 1. Comparing with Al2O3 bar support, a diminution in the total acidity was observed in the In–Al2O3 doped supports (0.75 to 0.64 mmol NH3/g.). The diminution of Al3+ in pentahedral coordination observed by NMR-MAS spectroscopy could be the cause of the diminution in the total acidity in the In–Al2O3 doped supports. On the other hand, in Pt/In–Al2O3 doped catalysts, the acidity was smaller and values ranging between 0.55 and 0.43 mmol NH3/g were reported. This additional diminution in the total acidity in catalysts impregnated with platinum can be explained by a selective deposit of platinum particles over the acidic sites of the support. A charge transfer from the metal particles to the support is then produced and platinum acquires electron deficiency (metal support interaction) [22]. For coimpregnated Pt–In/Al2O3–I catalyst, the diminution in acidity was dramatic (0.13 mmol NH3/g). This result can be expected since both platinum and indium were added over the alumina surface by the impregnation method and the deposit of both metals over the acidic sites must be more important. Certainly, the preparation method sol–gel or impregnation will define the catalytic properties of the solids. A different behavior for the n-heptane conversion over the Pt–In/Al2O3–I catalyst can be expected, as it was established that such a reaction is a bifunctional reaction requiring metallic and acid sites.
The catalytic activity for the n-heptane conversion was determined for all the platinum catalysts and the results are reported in Table 2. The Pt/Al2O3 catalyst showed the lowest activity in the steady state (after 14 h in stream). Meanwhile, for the Pt/In–Al2O3 doped catalysts and for the Pt–In/Al2O3–I coimpregnated catalyst, a higher activity and lower deactivation rate were observed after 40 h in stream (Fig. 5).
The conversion of n-heptane is accepted as a bifunctional reaction, as has been reported by Passos et al., using Pt–In/Al2O3 coimpregnated catalysts [10]. The selectivity to aromatic compounds (toluene and benzene) or to the C5–C7 fraction and hydrocracking products (C1–C4) will depend on the equilibrium between the acidity of the support and of the platinum properties. In the Pt/In–Al2O3 doped catalysts, the acidity was spread over a small range (from 55 to 43 mmol NH3/g) and it is practically constant. On the other hand, any dilution effect of the platinum conglomerates by the presence of indium can be mentioned in doped catalysts, since all of them showed a comparable metal dispersion (from 38 to 33%D).
The differences in activity and selectivity observed in the n-heptane conversion over Pt/Al2O3 or Pt/In–Al2O3 doped catalysts must be due to the support effects on the platinum particles. We suggest that such effects are a consequence of the selective deposit of the platinum over the acid sites of the support and to the decrease of the metallic character by the support effect. Hence, metallic functions as dealkylation [23] of toluene must be affected strongly over Pt/In–Al2O3 doped catalysts (Table 2).
The variation in the catalytic properties as an effect of modification of the support has been reported on rhodium supported over silica and alumina [24], Pt/HY [22], and Pt/ZrO2–Al2O3 catalysts [25]. The effect of the nature of the support in such catalysts was due to the formation of platinum electron-deficient particles; this effect is very different to the dilution metallic effects observed in bimetallic alloyed catalysts, as has been reported for Pt–Pb/La–Al2O3, Pt–Sn/SO2-Al2O3 catalysts [26, 27].
The selectivity of the coimpregnated Pt–In/Al2O3–I catalyst showed high yield in toluene and benzene (Table 2). The modification of the intrinsic platinum catalytic properties shown by the indium-doped alumina was not observed in the coimpregnated catalysts. Thus, we prepared Pt/In–Al2O3 doped catalysts in which the origin of the modifications in the activity and selectivity for the n-heptane conversion is very different to that reported for alloyed coimpregnated bimetallic catalysts.
4 4 Conclusions
It is assumed that the incorporation of indium to alumina gel induces modifications in textural, structural, and catalytic properties of Pt/In–Al2O3 sol–gel doped catalysts. High specific surface areas and narrow pore size distribution were obtained by doping alumina gel with indium acetylacetonate. The incorporation of In3+ to the alumina gel disturbs the formation of pentahedral coordinated aluminum, as has been suggested by 27Al NMR-MAS analysis spectra. On the other hand, the selectivity for the n-heptane conversion over Pt/Al2O3 and Pt–In/Al2O3 coimpregnated catalysts was modified strongly when Pt/In–Al2O3 sol–gel doped catalysts were used, suggesting that there are important support effects over platinum particles. The modification of the platinum metallic properties by the influence of the support was demonstrated by a diminution in the formation of benzene in the n-heptane conversion.
These results show a promising alternative for the production of ecological benzene-free gasolines in the reforming units, since the modifications on Pt/In–Al2O3 doped catalysts prepared by sol–gel method avoid the formation of benzene as a product in the n-heptane conversion.
References
Srinivasan R, Davis BH (1992) Platinum Met Rev 36:151
Carnevillier C, Epron F, Marecot P (2004) Appl Catal A: Gen 275:25
Ronning M, Gjervan T, Prestvik R, Nicholosn DG, Holmen A (2001) J Catal 204:292
Audo C, Lambert JF, Che M, Dillon B (2001) Catal Today 65:157
Biloen P, Helle JN, Verbeek H, Dautzemberg FM, Sachtler WMH (1980) J Catal 63:112
Wotsch A, Pirault-Roy L, Levard J, Guerin M, Paal Z (2002) J Catal 208:490
Homs N, Llorca J, Riera M, Jolis J, Fierro JLG, Sales J, de la Piscina PR (2003) J Mol Catal A 200:251
Yuhan S, Songying C, Shaoyi P (1991) React Kinet Catal Lett 45:101
Padmavathi G, Chaudhuri KK, Rajeshwer D, Rao GS, Krishnamurthy KR, Trivedi PC, Hathi KK, Subramanyam N (2005) Chem Eng Sci 60:4119
Passos FB, Aranda DAG, Schmal M (1998) J Catal 178:478
Passos FB, Schmal M, Vannice MA (1996) J Catal 160:118
Passos FB, Shmal M, Vannice MA (1996) J Catal 160:106
Gomez R, Bertin V, Ramirez MA, Zanudio T, Bosch P, Schifter I, Lopez T (1992) J Non-Cryst Solids 147–148:748
Gomez R, Bertin V, Bosch P, Lopez T, Del Angel P, Schifter I (1993) Catal Lett 21:309
Gomez R, Bertin V, Lopez T, Ferat G, Schifter I (1996) J Mol Catal A: Gen 109:55
Gomez R, Lopez T, Bertin V, Silva R, Salas P, Schifter I (1997) J Sol–Gel Sci Technol 8:847
Del Angel G, Bonilla A, Navarrete J, Figueroa EG, Fierro JLG (2001) J Catal 203:257
del Angel G, Bonilla A, Peña Y, Navarrete J, Fierro J-L-G, Acosta DR (2003) J Catal 219:63
Gomez R, Sanchez J, Silva R, Lopez T (1996) React Kinet Catal Lett 59:247
May M, Asomoza M, Lopez T, Gomez R (1997) Chem Mater 9:2616
Wang JA, Bokhimi X, Morales A, Novaro O, Lopez T, Gomez R (1999) J Phys Chem B 103:299
Gallezot P, Dakta J, Massardier J, Primet M, Imelik B (1977) In: Proceedings of the VI International Congress on Catalysis. Chemical Society, London, p 696
Barbier J (1986) Appl Catal 23:225
Del Angel G, Coq B, Figueras F, Fuentes S, Gomez R (1983) New J Chim 7:173
Castillo S, Moran-Pineda M, Gomez R (2001) Catal Commun 2:295
Del Angel G, Torres G, Bertin V, Gomez R, Moran-Pineda M, Castillo S, Fierro JLG (2006) Catal Commun 4: 232
Corro G, Velasco A, Montiel R (2001) Catal Commun 2:369
Acknowledgments
We acknowledge CONACYT for the financial support for Project No. 32274. V.R. thanks CONACYT for a fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez-González, V., Gómez, R., Moscosa-Santillan, M. et al. Synthesis, characterization, and catalytic activity in the n-heptane conversion over Pt/In–Al2O3 sol–gel prepared catalysts. J Sol-Gel Sci Technol 42, 165–171 (2007). https://doi.org/10.1007/s10971-007-1540-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-007-1540-z