Abstract
The concentrations of 226Ra and 222Rn were measured in ground water samples of Davanagere district, Karnataka state, India using emanometry technique. Activity of 226Ra and 222Rn in groundwater varied from 15.6 ± 3 to 68.9 ± 5 mBql−1 and 37 ± 4 to 245 ± 8 Bql−1 respectively. More than 53% of the water samples showed higher radon concentration compare to standard safe limit of 100 Bql−1 prescribed by WHO. Effective dose to the public due to 222Rn in water was found to vary from 0.15 to 1.00 mSvy−1 with an average of 0.49 mSvy−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radium is a naturally occurring radionuclide formed by the radioactive decay of uranium and thorium isotopes in the environment. During weathering of rocks, radium can move in the particulate phase to be transported and deposited in nearby soil. When ground water moves through rocks and soil, radium isotopes will be dissolved in water and transported with it and can be found at low levels virtually in all types of soils, water, plants and animals. Radium isotopes are chemically similar to calcium and are absorbed by plants and passed on through the food chain to human beings. When ingested, about 80% of the radium tends to accumulate in the bones [1]. Surface and groundwater are the main sources through which humans fulfil their water requirements and among these, groundwater contains higher concentrations of radioactive elements [2]. The radionuclide present in drinking water contributes significantly to the amount of dose received by living beings.
222Rn is the radioactive decay product of 226Ra, being an inert gas, it can get transported to the open atmosphere through soil, water, rocks and eventually in to the human body through inhalation and ingestion. 222Rn gets released from water to indoor air by household activities, such as showers, cleaning, washing, and so on. Radon gets into human beings through inhalation and affects lungs and related organs [3]. Ingestion add dose to the stomach, intestine, and bones. Cancer risk caused by inhalation of radon is greater than that caused by consumption of water and there is a strong correlation between radiation exposure and health hazards among the population in a given environment depending upon the concentration of radioactive materials [4,5,6,7].
226Ra and 222Rn are the important radionuclide from the radiological significance due to their high level of toxicity. Among all the natural sources of radiation exposure, 222Rn along with its daughter nuclei contribute more than 50% of the total population-weighted annual effective dose [8]. Since 222Rn poses a serious health risk to humans it becomes very essential to study about its presence in water.
A study of the distribution of 226Ra and 222Rn activity in the natural water is important in evaluating the radiation exposure to the population. A large number of studies have been carried out in different parts of the world about concentration of these radionuclide in water [9,10,11,12,13]. However no such studies have been carried out in the area of Davanagere district, Karnataka state, India. This study is a first attempt to estimate the activity of radon and radium in groundwater and to estimate the inhalation and ingestion dose to the population of Davanagere district, Karnataka state, India.
Study area
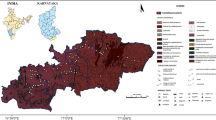
The study area, Davanagere district, is located in mid-eastern region of Karnataka state, India having a geographical area of 5931 km2 and the lithology map of the region is shown in Fig. 1. The rock formation is weathered (vary from 4.0 to 36.74 m) and fractured comprising of granites, gneisses and schist belonging to peninsular gneissic group. Geological formations in the study area are placed under major sedimentary component called younger schist belt of Dharwar type [14]. Major part of the region lies in Krishna river basin, drained by Tunga Bhadra and Chikka Hagari rivers. Southwest monsoon contributes about 58% of the total rainfall while northeast monsoon contributes about 22% in the district. The recharge of ground water occurs through precipitation and irrigation activities. Hydrogeology of the study area is given in Table 1.
Lithology map of Davanagere district [14]
The occurrence of radionuclides varies from place to place on the earth depending on local geology and geography. The data generated through the present studies is expected to provide a clear comprehensive picture of radioactive elements present in this region and the type of geology responsible for the observed level of radioactive elements in the environmental matrix.
Experimental methods
Measurement of 222Rn concentration in water samples
The concentration of 222Rn in ground water samples were evaluated using Smart Radon Monitor (SRM), an instrument developed by BARC, Government of India and fabricated by Para Electronics Manufacturing Division [15,16,17]. SRM measures alpha from radon and its decay products by using ZnS (Ag) based scintillation cell which is having a volume of 150 cm3. Experimental set-up for the measurement of radon in water samples is shown in Fig. 2. The samples were collected in air tight bottles of volume 500 ml made of material of lower permeability. Care was taken such that no bubbles were formed inside the bottle. Sampling time and GPS of locations were noted and the measurements were done within 4–5 h. The analysis of the sample was done following the standard test method for radon in drinking water given by American Society for Testing and Measurements (ASTM) [18,19,20]. Out of 500 ml of the water sample, 60 ml was transferred to the sampling holder of the SRM. Dissolved radon in the sample was transferred to the scintillation cell by bubbling air through the sample using a specially designed pump. On agitating water, the dissolved radon gets released and enters into scintillation cell.
In the SRM, the bubbled air is made to pass through a progeny filter before entering into the scintillation cell to eliminate 222Rn progeny and 220Rn progeny. Alpha particles emitted from 220Rn were also eliminated using a 220Rn discriminator due to short half-life of, 220Rn daughter, 216Po (0.14 s). The scintillations produced due to the alpha particles emitted by radon and its decay products were measured with the help of a photomultiplier tube which is coupled to counting electronics. The SRM microprocessor is associated with indigenous smart algorithm which automatically compensates the background counts due to residual decay product of 222Rn. The efficiency of scintillation cell associated with SRM is 74%.
The equation to estimate the Radon concentration in water (CRnw) from the concentration of 222Rn measured in air (Cair) using SRM is, [15,16,17].
where k is the partition coefficient of radon gas in water with respect to air with value equivalent to 0.25. Vair and VRnw are the total volume of air gap and volume of the water sample respectively.
The annual mean effective dose due to inhalation and ingestion of 222Rn in water was calculated by using the parameter established in UNSCEAR—2000 and WHO—2011 reports [8, 21]
The inhalation dose is calculated using the following relation.
where Din is the effective dose for inhalation, CRnw is the 222Rn concentration in water (kBqm−3), Raw is the ratio of 222Rn in air to water (10−4), F is the equilibrium factor between 222Rn and its progeny (0.4), I is the average indoor occupancy time per individual (7000hy−1) and DCF is the dose conversion factor for 222Rn exposure 9 nSv (Bqhm−3)−1.
The ingestion dose is calculated using the following relation.
where Dig is the effective dose for ingestion, CRnw is the 222Rn concentration in water (kBqm−3), VW is the weighed estimate of water consumption and EDC is the effective dose coefficient for ingestion (3.5 nSvBq−1). IAEA dose coefficients and prescribed water intake rates for different age groups were used in the estimation of effective dose [22].
A demographic survey on water intake by the population was conducted in the 5 taluks of Davanagere district. The average daily metabolic intake rate of water by men, women and children in the study area were found to be 1250 ml, 1000 ml and 1500 ml respectively. The Davanagere district population consists of 642,913 men, 621,092 women and 499,188 children [23, 24]. Thus weighted average of water intake by the population of Davanagere district was considered to be 450 ly−1. WHO recommends water consumption of 730 ly−1 and UNSCEAR has considered weighted estimate of water consumption of 60 ly−1 for dose estimation. In the present study the ingestion dose to the population of Davanagere district was estimated by considering water intake of both 450 ly−1 and 730 ly−1.
Measurement of 226Ra concentration in water samples
Generally the concentration of 226Ra in water samples will be lower than the detection level of the counting system. Therefore pre-concentrated solution of water sample was used for the 226Ra analysis. In the present study 20 l of water sample was collected in a polythene air tight prewashed container. The samples were acidified with HNO3 to avoid the adsorption of the actinides on the walls of the container. The samples were filtered through a whatman filter paper No. 42 prior to the analysis. The water sample was co-precipitated with MnO2, then pre-concentrated by evaporation and chemical method to estimate the activity of 226Ra. Pre concentrated samples of about 70 ml was transferred in to the radon bubbler and initially whatever the radon in the solution was removed by aeration with the help of a low suction pump. After aeration is completed the bubbler is sealed and allowed to stand for radon to build up and accumulate in the solution [25,26,27]. The schematic diagram of radon bubbler is shown in Fig. 3. The build-up period is determined by the expected radium content and is generally 7 half lives of radon which is nearly about 21 days. The accumulated radon in the bubbler was transferred to the evacuated scintillation cell (150 cm3) through rubber tubing, which was well sealed from the outside atmosphere. The dissolved radon in the water sample gets desorbed due to agitation and enters into the scintillation cell by vacuum transfer technique. Alpha activity in the scintillation cell due to radon and its progeny was measured using an alpha probe specially designed for this purpose coupled with a counting system.
The activity concentration of 226Ra dissolved in the solution is given by [25,26,27].
where, D = Counts above background, V = Volume of water (70 ml), E = Efficiency of the scintillation cell (74%), λ = decay constant for radon (2.098 × 10–6 s−1), T = Counting delay after sampling, t = Counting duration (s) and θ = build up time in the bubbler (s).
Ingestion dose due to intake of 226Ra through the drinking water pathway was calculated using IAEA dose coefficients [22]. The annual ingestion dose was calculated by the Eq. (5) [8].
where, DRa is the ingestion dose due to 226Ra (Svy−1), CRa is the mean concentration of 226Ra in the water (Bql−1), VW is the weighed estimate of water consumption (ly−1), DCF is the dose conversion factor for a particular radionuclide and for a specific age group (Sv Bq−1).
Results and discussion
The activity concentration of 222Rn and estimated values of ingestion and inhalation dose to the population in the study area are shown in Table 2. 222Rn concentration in groundwater varies from 37 ± 4 to 245 ± 8 Bql−1 with a GM of 103 ± 6 Bql−1. The total annual effective dose due to inhalation and ingestion varied from 0.15 to 1.00 mSvy−1 with an average of 0.49 mSvy−1. The distribution of radon concentration in drinking water samples of Davanagere district shows a wide variation and it is shown in Fig. 4. The maximum radon concentration of 245 ± 8 Bql−1 was found in Harapanahalli town. Taluk wise distribution of radon concentration in drinking water of Davanagere district is shown in Fig. 5. Samples collected in Jagalur taluk have the highest average 222Rn concentration in water with a value of 179.6 ± 6 Bql−1. This can be due to excessive mining of manganese and iron ore in that region. The area also comprises of Granitic-gneisses and schists. Granite rocks were found to have higher quantities of radioactive elements such as 226Ra and 232Th thus groundwater near granitic rocks have higher radon concentration [8, 28,29,30,31].
Environmental radiation protection agencies like USEPA [32], UNSCEAR [33], WHO [34] have given maximum permissible limits on radon concentration in drinking water. United States Environmental Protection Agency (USEPA), have set a Maximum Contamination Limit (MCL) for radon in water to be 11.1—148 Bql−1. About 53% of the samples are above the WHO [34] recommended value of 100 Bql−1. This may be because radioactive elements dissolve in water during transportation near the rocks (like granites) and soil rich in radionuclide. Uranium mineralization, geology of the study area, type of soil and rock formation along with the depth of the borewell were also responsible for the variations in radon concentration. The effective dose from ingestion and inhalation of radon concentration in water varies from 0.15 to 1.00 mSvy−1 with an average of 0.49 mSvy−1. The average effective dose value was higher than the safe limit of 0.1 mSvy−1 recommended in WHO report [34].
The concentration of 226Ra and 222Rn in water sample of selected locations in Davanagere district is shown in Table 3. Weighted average consumption of 450 L of water in a year was considered for dose estimation. 226Ra concentration in water varies from 15.6 ± 3 to 68.9 ± 5 mBql−1 with a GM of 37.1 ± 4 mBql−1. Higher concentration of 226Ra in ground water is observed in Harapanahalli taluk, Gundagatti village due to its lithological conditions and presence of genesis and schists types of rocks. The limit for 226Ra concentration in drinking water reported by WHO is 1 Bql−1 [34]. All the samples of the study area shows 226Ra concentration below this limit. Ingestion dose for an adult population due to intake of 226Ra through drinking water was found to be varied from 1.99 to 8.80 µSv y−1 with an average of 5.35 µSv y−1.
The variation of 226Ra with 222Rn concentration in ground water is as shown in Fig. 6. A good correlation between 226Ra and 222Rn concentration was observed with a Pearson’s r value of 0.786. The concentration of 222Rn in ground water depends on the concentration of its parent 226Ra, in the underlying rock [35]. The short-life of 222Rn (3.82 days) together with the slow rate of migration of ground water allows the 222Rn in solution to be in approximate secular equilibrium with the 226Ra in the local rock. Radon concentrations in water have been known to be high in most granite and in high-grade metamorphic rocks, whereas less metamorphosed rocks have somewhat less 226Ra. In ground water higher 226Ra and 222Rn concentrations were found in granitic and orthoclase types of rocks and very low concentration is observed in staurolite type of rocks [36]. The radon concentration in ground water also depends on several other factors such as pressure, earth quake actions and rainfall [37].
Conclusion
A systematic study of 226Ra and 222Rn concentration in groundwater of Davanagere district has been carried out during 2019–20. Radon concentration in water varied from 37 ± 4 to 245 ± 8 Bql−1 with a GM of 103 ± 6 Bql−1. The total effective dose from ingestion and inhalation of radon concentration in water varies from 0.15 mSvy−1 to 1.00 mSvy−1 with an average of 0.49 mSvy−1. The radium concentration ranges from 15.6 ± 3 mBql−1 to 68.9 ± 5 mBql−1 with GM of 37.1 ± 4 mBql−1. The ingestion dose for the adult population due to intake of 222Ra in water varied from 1.99 to 8.80 µSv y−1 with an average of 5.35 µSv y−1. A good correlation between 226Ra and 222Rn concentration is observed with a Pearson’s r value of 0.786.
References
UNSCEAR (1982) Ionizing radiation: sources and biological effects. United Nations scientific committee on the effects of atomic radiation
Choubey VM, Bartarya SK, Saini NK, Ramola RC (2001) Impact of geohydrology and neotectonic activity on radon concentration in groundwater of intermontane Doon Valley, Outer Himalaya India. Environ Geol 40(3):257–266
Vinson DS, Campbell TR, Vengosh A (2008) Radon transfer from groundwater used in showers to indoor air. Appl Geochem 23(9):2676–2685
Mays CW, Rowland RE, Stehney AF (1985) Cancer risk from the lifetime intake of Ra and U isotopes. Health Phys 48(5):635–647
Sethi TK, El-Ghamry MN, Kloecker GH (2012) Radon and lung cancer. Clin Adv Hematol Oncol 10(3):157–164
Henshaw DL, Eatough JP, Richardson RB (1990) Radon as a causative factor in induction of myeloid leukaemia and other cancers. The Lancet 335(8696):1008–1012
Fornalski KW, Adams R, Allison W, Corrice LE, Cuttler JM, Davey C, Welsh JS (2015) The assumption of radon-induced cancer risk. Cancer Causes Control 26(10):1517–1518
UNSCEAR (2000) Annex B: Exposures from natural radiation sources, United Nations Scientific Committee on the Effects of Atomic Radiation, United States
Inacio M, Soares S, Almeida P (2017) Radon concentration assessment in water sources of public drinking of Covilhas county, Portugal. J Radiat Res Appl Sci 10(2):135–139
Prasad M, Kumar GA, Sahoo BK, Ramola RC (2018) A comprehensive study of levels and associated radiation dose in Himalayan ground water. Acta Geophys 66(55):1223–1231
Ezzulddin SK, Mansour HH (2020) Radon and radium activity concentration measurement in drinking water resources in Kurdistan Region-Iraq. J Radioanal Nucl Chem 324(3):963–976
Nagaraja K, Prasad BSN, Chandrashekara MS, Paramesh L, Madhava MS (2006) Inhalation dose due to radon and its progeny at Pune Indian. J Pure Appl Phys 44:353–359
Seminsky KZ, Seminsky AK (2019) Radon concentration in groundwater sources of the Baikal region (East Siberia, Russia). Appl Geochem 111:104446
Ground Water Information Booklet Davanagere District, Karnataka, Central Groundwater Board, Ministry of Water Resources, Government of India (2008)
Gaware JJ, Sahoo BK, Sapra YS, Mayya, (2011) Development of online thoron monitoring systems for occupation and general environments. BARC News Letter 318:45–51
Singh P, Singh P, Sahoo BK, Bajwa BS (2016) A study on uranium and radon levels in drinking water sources of a mineralized zone of Himachal Pradesh, India. J Radioanal Nucl Chem 309(2):541–549
Rani S, Kansal S, Singla AK, Mehra R (2021) Radiological risk assessment to the public due to the presence of radon in water of Barnala District, Punjab India. Environ Geochem Health 43(12):5011–5024
ASTM (1998) American society for testing and measurements, Standard test method for radon in drinking water, D5072–98
Rajesh BM, Chandrashekara MS, Nagaraja P, Paramesh L (2012) Studies on radon concentration in aqueous samples at Mysore city India. Radiat Protect Environ 35(1):9–13
Jobbagy V, Altzitzoglou T, Malo P, Tanner V, Hult M (2017) A brief overview on radon measurements in drinking water. J Environ Radioact 173:18–24
WHO (2011) World Health Organization, Guidelines for Drinking Water Quality-4th edition, Radiological aspects: 203–217
IAEA (2011) International Atomic Energy Agency, Radiation Protection and Safety Of Radiation Sources: International Basic Safety Standards, in IAEA Safety Standards, Interim ed. Austria
Census of India (2011) Karnataka, Davanagere district Census Handbook
National Informatics Centre, Davanagere district (2017), Government of India: statistical report
Raghavayya M, Iyengar MAR, Markose (1980) Estimation of 226Ra by emanometry. Bull Radiat Protect 11(2):33–35
ASTM (2005) American Society for testing and measurements, Standard test method for Radium-226 in water, D3454–05
Maharana M, Eappen KP, Sengupta, D (2010) Radon emanometric technique for 226Ra estimation. J Radioanal Nucl Chem 285(3):469–474
Sannappa J, Chandrashekara MS, Sathish LA, Paramesh L, Venkataramaiah P (2003) Study of background radiation dose in Mysore city, Karnataka State India. Radiat Measure 37(1):55–65
Porntepkasemsan B, Srisuksawad K (2008) Assessment of 226Ra age-dependent dose from water intake. Appl Radiat Isot 66(11):1654–1656
Institute of Medicine (2005) Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. The National Academies Press
Strain CD, Watson JE, Fong SW (1979) An evaluation of radium-226 and radon-222 concentrations in ground and surface water near a phosphate mining and manufacturing facility. Health Phys 37:779–783
USEPA (1991) United States Environmental Protection Agency (1991) Federal Register 40 Parts 141 and 142: National primary drinking water regulations for radionuclides: proposed rules, 56: 33050–33127
UNSCEAR (2019) Annex B: Lung cancer from exposure to radon, United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and effects of ionizing radiation. New York: United Nations
WHO (2004) World health organization, guidelines for drinking water quality. Radiol Aspects, Geneva 1(3):1–203
Faweya EB, Agbetuyi OA, Talabi AO, Adewumi T, Faweya O (2021) Radiological Implication of 222Rn Concentrations in Waters from Quarries environs correlation with 226Ra concentrations and rocks Geochemistry. Arab J Geosci 14(11):1–15
Kozlowska B, Hetman A, Zipper W (1999) Determination of 222Rn in natural water samples from health resorts in the Sudety mountains by the liquid scintillation technique. Appl Radiat Isot 51(4):475–480
Wakita H, Igarashi G, Nakamura Y, Sano Y, Notsu K (1989) Coseismic radon changes in groundwater. Geophys Res Lett 16(5):417–420
Acknowledgements
Authors thank DST-SERB Govt of India for providing financial support to carry out this work. The authors express their profound gratitude to Prof. P. Venkataramaiah, Former Vice-chancellor, Kuvempu University and Professor (Retired), Department of Studies in Physics, University of Mysore, Mysore, for useful discussions and constant encouragement throughout the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hidayath, M., Chandrashekara, M.S., Rani, K.S.P. et al. Studies on the concentration of 226Ra and 222Rn in drinking water samples and effective dose to the population of Davanagere district, Karnataka state, India. J Radioanal Nucl Chem 331, 1923–1931 (2022). https://doi.org/10.1007/s10967-022-08240-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08240-1