Abstract
In this work, the impacts of TENORM (Technologically Enhanced Naturally Occurring Radioactive Materials) from fertilizers on soil and vegetables were estimated. We investigated both the activity concentration of the natural radionuclides and the annual effective dose rate due to the ingestion of vegetables in the crops using fertilizers at the agricultural zone of Hoc Mon, Ho Chi Minh City, Vietnam. The results show that there have not yet been signs of radioactive residues from using conventional fertilizers in agricultural land after a crop at the surveyed area and time. The radiological impact of surveyed vegetables was negligible to the public health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radioactive isotopes are found all over the human environment: in fossil fuel, soil, rocks, water, air, phosphate ore, vegetation, and within the human body itself. The radioactive substances and radiation that can reach the Earth are also caused by the interaction of cosmic rays with elements in the atmosphere. Also, with the development of the global economy, the advancement of nuclear technology has created an enhanced radiation background through atomic weapons tests, the operation of nuclear reactors developed to produce electricity, radioactive isotope technologies, etc.
Fertilizers are products from phosphate rock, which contain relatively high concentrations of natural radionuclides. Therefore, the massive amounts of consumption every year in crops can redistribute radioactive trace elements in soils. Vegetables are terrestrial foods. The migration of naturally occurring radioactive material (NORM) and technology-enhanced naturally occurring radioactive material (TENORM) in soils could enhance radioactive nuclides in vegetables. People may be exposed through the ingestion of vegetables that contains radionuclides resulting from fertilizers and soils. Radioactive nuclides can contaminate plants in many different ways. In the process of growing, plants will receive nutrients from the surrounding environment: soil, groundwater, rainwater, fertilizer. Therefore the radioactive nuclides will be accumulated in the plant. Similarly, the leaf surface may be contaminated by depositing radionuclides from the atmosphere or by irrigating contaminated water.
The previous studies of the natural radioactivity in phosphate rock, in NPK fertilizers, showed that the soil might be contaminated with both natural radionuclides and micronutrients [1, 2]. Indeed, the enhancement in natural radioactivity level for soils and vegetables due to the usage of phosphate fertilizers in agricultural lands were found in Bolca et al. [3]. The natural radioactivity, dose assessment, and uranium uptake of some crops in Khan Al-Zabeeb, Jordan was studied by Al-Kharouf et al. [4].
In 2014, Asaduzzaman et al. studied the transport of radioactive isotopes of 226Ra, 232Th, 40 K, and 88Y from the soil into root vegetables in some areas of Malaysia [5]. In 2016, Al-Hamarneh and his colleagues studied radioactivity and transfer factor (TF) of 226Ra, 234U, and 238U isotopes from soil to plants for 13 crops at the farms in the North West of Saudi Arabic [6].
In this work, the natural radionuclide activity from fertilizers and their residues on soil and vegetables after crops at the agricultural zone of the Hoc Mon District, Ho Chi Minh City, Vietnam, were evaluated. Besides, the annual effective doses due to the ingestion of these vegetables, and the radiological impacts of vegetable ingestion on humans were assessed at the surveyed zone.
Materials and method
Materials
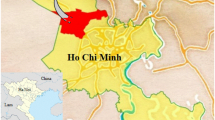
Vegetables were grown at the farm of Xuan Thoi Thuong zone, Hoc Mon District, Ho Chi Minh City, Vietnam (Fig. 1). Xuan Thoi Thuong is located in the southwest of the Hoc Mon District. Xuan Thoi Thuong has an area of 18.09 km2 and is a fertile land with many canals and water sources, which are favourable for growing fresh vegetables. It is one of the extensive vegetable baskets of Ho Chi Minh City. Therefore, it is necessary to evaluate the quality of fresh vegetables thorough evaluation for the radioactivity concentration of natural radionuclides, which are still accumulated in vegetables after harvesting.
To evaluate the effects of radioactivity in fertilizer on soil and vegetable after harvesting, we cultivated Ipomoea Aquatica on 13 plots. Table 1 shows different types of fertilizer for each (F1 to F12 and F13 for non-use of fertilizers was used to be the reference plot). The same fertilizer amount of 0.1 kg/m2 was used for each plot. Soil samples at 0–20 cm from the surface were selected because the root density is usually found in these soil layers for leafy vegetables [7]. At each plot, five topsoil samples were collected and mixed for a representative sample. They were taken from the respective plots before planting (B) and after harvesting (A) (denoted by S1 to S13). The Ipomoea Aquatica samples of 10 kg were collected at the 13 plots (denoted by V1 to V13). To ensure statistics in evaluating the radioactivity level for the 13 types of the concerned fertilizer, three fertilizer samples of 200 mg from the same type were mixed to have a representative sample of each kind of fertilizer. These samples were prepared and analyzed by using a gamma spectrometer with an HPGe detector to evaluate the natural radioactivity of 238U, 226Ra, 232Th, 210Pb, and 40 K.
To evaluate the effective dose rate from internal exposure due to ingestion of vegetables which were cultivated in the surveyed zone, the vegetable samples of turnip (denoted by Tur), basil (Bas), amaranthus tricolor (Amat), Ipomoea aquatica (Ipo), amaranthus (Ama), mustard (Mus), serrate leaf (Ser), Malabar spinach (Mas), sui choy (Sui), jute plant (Jute), sweet potato leaf (Spo) which are commonly consumed in Vietnamese meals were studied. The samples of these vegetables were collected after a crop, and each sample had a fresh weight of about 10 kg.
The edible parts, including the leaf and stem of each sample of vegetables, were washed, dried at the room temperature, ashed at 450 °C for 24 h. Each sample of soil or fertilizer was dried at the room temperature, crushed to their particle sizes of less than 0.2 mm. Then samples were dried at 105 °C, packed in cylinder beakers, and sealed for about 40 days to ensure the secular equilibrium between 226Ra radionuclide and its short-lived decay products [8].
Instrumentation and calibration
Activity concentrations of 238U, 226Ra, 232Th, and their daughters and 40 K radionuclides from these samples were measured by the gamma spectrometer with high purity germanium (HPGe) detector of ORTEC Industries Inc. (GMX35 P4-70). The activity concentration of a specific radionuclide was determined using the relation given in Eq. (1).
where A is the sample activity concentration on the sampling date (Bqkg-1), S is the net peak area, \(\varepsilon (E)\) is full energy peak efficiency of the detector, f is the gamma yield of the E gamma energy under consideration, m is the mass of the sample (kg), and t is the live collection time (s), Kc is the correction factor for the nuclide decay during counting and Kw is the correction factor for the nuclide decay from the time the sample was obtained to the start of acquisition [9].
Minimum Detectable Activity—MDA (Bq kg−1) values were also calculated for every interested energy line as follows:
where \(L_{D} = 2.71 + 4.66\sqrt B\) is the detection limit for a confidence interval of 95%; B is the continuum under the peak [9].
The Full Energy Peak Efficiency (FEPE) of the detector was calibrated by measurements of gamma spectra emitted from the radionuclides of uranium, thorium series, and potassium in the certified IAEA soil standard samples of RGU1, RGTh1, and RGK1. The self-absorption effect of gamma rays caused by the difference of composition and density between analyzed samples and standard samples were corrected by using the efficiency calculation software -Angle 3.0 [10]. The true coincidence summing effects were corrected by the CCCC code [11].
The activity of radionuclide was estimated using the acquiring gamma spectra from itself or via its direct daughter radionuclides (taking the weighted average of activities). Briefly, these are the 46.5 keV gamma for 210Pb; 63.38 keV gamma (234Th) and 1001 keV gamma (234mPa) for 238U; 295 keV and 352 keV gammas (214Pb), and 609 keV gamma (214Bi) for 226Ra; 338 keV, 795 keV and 911 keV gammas (228Ac) for 232Th; the 212Pb, 212Bi, 40 K activities were estimated by their 238 keV, 727 keV and 1460 keV gammas respectively. The recorded activity of 232Th was based on the assumption that 232Th is in secular equilibrium with its progenies, which is often not true, particularly in the case of the agricultural land.
The MDA values were also calculated for every interested gamma energy. The calculated values of activity were compared with these respective MDA values before giving the final results [12]. The standard deviations of the activity concentration (A) were calculated from the error propagation of S-the net peak area, \(\varepsilon (E)\)-full energy peak efficiency of the detector, f-the branching ratio of the E gamma energy under consideration, m-the mass of the sample (kg) given in Loan et al. 2018b [2].
Radium equivalent activity-Ra eq (Bq kg −1 )
The radium equivalent activity of the sample containing different levels of 226Ra, 232Th, and 40 K nuclides are estimated as follows:
where ARa, ATh, and AK are activity concentrations (Bq kg−1) of 226Ra, 232Th (228Ac), and 40 K nuclides respectively in the samples. The maximum value of Raeq must be less than 370 Bq kg−1 to keep the absorbed dose of less than 1.5 mGy y−1 [13].
The annual effective dose due to ingestion of terrestrial food
The annual effective dose due to ingestion of terrestrial food, f, containing radionuclide, i, is given by Saueia et al. [14].
EV is the annual effective dose (Sv y−1) due to ingestion of terrestrial food; CV, i is activity concentration of radionuclide i in the edible part of plants (Bq kg−1); Uv is ingestion rate (kg y−1); FCDing, i is the dose conversion factor (Sv Bq−1). The methodology of UNSCEAR, 2017 [15] employs dose coefficients for an adult member of the public and the committed effective doses to 70 years of age per unit intake of radionuclides given by ICRP, 2012 [16].
Note: The upper limit of the ingestion rate of vegetables for Vietnamese people is 272 g day−1 (per capita) [17]. The values of FCDing, v are given by UNSCEAR, 2017 [15].
Results and discussions
Natural activity concentration in fertilizer samples
Table 2 presented the activity concentrations of 238U, 226Ra, 232Th (228Ac), 40 K, 210Pb in fertilizer samples. The results showed that there is a difference in radioactivity concentrations in 12 types of NPK chemical fertilizers. The variation depends on the existence of chemical elements such as N, P, K, and other nutrient content in the fertilizer samples. The activity concentrations of fertilizer samples vary from 1.2 Bq kg−1 to 598.6 Bq kg−1 for 238U, from 1.8 Bq kg−1 to 111.3 Bq kg−1 for 226 Ra, from 45 Bq kg−1 to 6391 Bq kg−1 for 40 K, from 0 Bq kg−1 to 11.7 Bq kg−1 for 232Th and from 0 Bq kg−1 to 50.4 Bq kg−1 for 210Pb. Similar results can also be found in previous work [18].
It is noticed that the activity concentrations of 238U in the surveyed fertilizer samples of NPK are mostly higher than the activity of 226Ra. It is attributed to the differences between production technologies, mining, processing, and origin of phosphate ore. The activity concentration of 226Ra will be significantly lost if the content of P2O5 is enriched by over 30% due to the chemical and thermal treatment and finish in phosphogypsum industrial waste [19].
The activity concentrations of 226Ra in the group of superphosphate (F2, F3, F12) are higher than these in other NPK fertilizers; their activities vary from 87.9 to 111.3 Bq kg−1, with the average activity of 97.0 Bq kg−1. This confirms the significant presence of radioisotope 226Ra these samples in phosphogypsum samples [19].
Activity concentration of natural radionuclides in the soil before planting and after harvesting
We investigated soil samples before planting and after harvesting to evaluate the effect of residual fertilizer on cultivation. The analytical results of the activity concentration of natural radionuclides were given in Table 3. Following that, the ratios of activity concentration in the soil before planting and after harvesting were estimated and shown in Fig. 2.
Figure 2 shows the ratios of these activity concentrations in the soil samples after harvesting and before planting using different fertilizers.
The analytical results showed that soil samples using different fertilizers after crops contained 238U, 226Ra, 232Th, 40 K, 210Pb radionuclides with the activity concentrations in the range from 0.5 to 1.5 times compared with the ones in soil samples before planting. The trend varies with the type of radioisotope and the absorption mechanism of the vegetable. In general, with a moderate amount of fertilizer, the soil after one crop has almost no radioactive residue from fertilizer, except for the soil sample at the S3 location, which is fertilized with phosphorus fertilizer (F3, Tables 1 and 2) containing a large amount of radioactive 226Ra (111.3 Bq kg−1) and 238U (46.5 Bq kg−1). As a result, the radioactivity concentrations of 238U, 226Ra, and 210Pb in soil samples at the S3 location increased from 45% (for 226Ra) to 73% (for 238U).
To evaluate the total effect of natural radioactivity in uranium, thorium series, 40 K, and 210Pb, the equivalent radium activity, Raeq for soil samples collected before planting and after harvesting were calculated, then the radioactive residues from the fertilizer on the surveyed agricultural land were estimated. The results are presented in Table 4.
The equivalent radium activities of the soil samples after harvesting is less than the limit of 370 Bq kg−1 given by UNSCEAR, 2000 [13]. It shows that there are no signs of radioactive residues in agricultural land due to fertilizer in the surveyed area.
Determination of the radioactivity of 238U, 226Ra, 232Th, 40 K, 210Pb in Ipomoea Aquatica samples (dry weight basis) using different fertilizers
Table 5 shows the natural radioactivity concentration in the Ipomoea Aquatica samples (in dry weight basis) which were grown in different fertilizer conditions ranged from 1.0 ± 0.2 to 10.9 ± 1.1 Bq kg−1 for 238U, from 2.0 ± 0.3 Bq kg−1 to 7.3 ± 1.8 Bq kg−1 for 226Ra, from 1.0 ± 0.6 Bq kg−1 to 4.2 ± 0.9 Bq kg−1 for 232Th, from 14.6 ± 2.8 Bq kg−1 to 34.3 ± 8.3 Bq kg−1 for 210Pb and from 1335 ± 41 Bq kg−1 to 3210 ± 97 Bq kg−1 for 40 K.
Among the radioisotopes, 40 K is the highest proportion and the second is 210Pb, while 232Th is found to be the lowest radioactivity concentration in Ipomoea Aquatica samples. It can be explained by the fact that potassium is a macronutrient for plants and plants absorb potassium from the soil to a certain extent, base to their metabolism. Thus, the highest radioactivity concentration of 40 K (3210 ± 97) Bq kg−1 in the V12 sample is explained that the fertilizer used in this location (F9, Table 1) was NPK (20-20-15), which has the highest potassium content (6391 ± 389) Bq kg−1. The lowest activity of 40 K (1335 ± 41) Bq kg−1 in the V1 sample is caused by the fact that there is no potassium content in the fertilizer sample used (F1, Table 1). Because of the high content of nitrogen and phosphorus, competitive absorptions in plants might occur between these elements and potassium available in the soil. In the meanwhile, the activity of 40 K (1629 ± 50) Bq kg−1 in V13 (no using fertilizer) shows that the plant absorbs potassium available in soils without any significant competition with other elements.
Also, the plant has an uptake of more 210Pb radioisotopes than the other three isotopes such as thorium, radium, and uranium. Although 210Pb exists only in F2 and F12 fertilizer samples, the 210Pb superiority in vegetables is explained by radioactive deposition from the air. Besides, there is an uptake of a lot of 210Pb from the soil through the root system.
The results also showed that in all of the surveyed Ipomoea Aquatica samples, plants absorbed more 226Ra than 238U and 232Th. It can be explained that 226Ra being a member of the 238U radioactive chain presents in all uranium-containing environments, and 226Ra usually exists in the form of water-soluble chemical compounds more than 238U. Finally, it makes the plant is easy to absorb 226Ra, which was also found in the work of Menzel and Verkhovskaya et al. [20, 21].
The activity concentration of natural radionuclides in common vegetables
To evaluate the effective dose rate from internal exposure due to the ingestion of vegetables which were cultivated in the surveyed zone, the vegetable samples which are commonly used in Vietnamese meals were grown using fertilizer as usual. The samples of these vegetables were then collected after a crop. The activity of natural radionuclides in these vegetables was analyzed. The values were given in Table 6.
The results showed that there is an accumulation of natural radionuclides in uranium-series, thorium series, 40 K, and especially of 210Pb in surveyed vegetable samples with the different activity concentrations. The activity concentration of 40 K radionuclides in vegetable samples showed higher than those of other radionuclides (about 100 times). The most important values of 148 ± 5; 148 ± 5; 130 ± 4; 122 ± 4 Bq kg−1 were found in basil, sweet potatoes, malabar spinach, mustard, respectively; the lowest value of 68 ± 2 Bq kg−1 was in the amaranthus sample. It is explained that potassium plays an important role in the growth of plants and therefore increases productivity and quality for crops.
The distribution of 40 K activity concentrations in different vegetables showed different uptakes of 40 K. The variation of activity concentration of 228Ac 212Pb, 212Bi, 208Tl in each vegetable sample proved the non-secular equilibrium between the 232Th radionuclides and their progenies of 228Ac 212Pb, 212Bi, 208Tl. The vegetables uptake more 228Ac than 212Pb, 212Bi, 208Tl. The highest 228Ac activity value of 2.36 Bq kg−1 is found in sweet potato leaf. The radioactivity concentration of 212Pb, 212Bi, 208Tl are also not the same in the different vegetables indicating there is an uptake competition for different isotopes in the same vegetable.
The 214Pb and 214Bi radioactivity concentrations are concentrated in basil, jute plant, and sweet potato leaf samples. The 214Pb and 214Bi radioactivity concentrations have relatively similar values in the same vegetable sample. In the meanwhile, the distribution of 238U in vegetables is not the same as the distribution of 214Pb, 214Bi radionuclides. The vegetables have different uptakes of 238U. The 238U activity has a high value of 0.83 Bq kg−1 and 0.66 Bq kg−1 for turnip sample and mustard, respectively. It indicates that a secular equilibrium does not happen between 238U radionuclides and their 226Ra progenies.
The 210Pb presents in most of the vegetables, such as turnips, amaranthus, jute plant, and sweet potato leaf with relatively low concentration with the highest value of 1.20 Bq kg−1 in turnips. It is explained by the deposition of the 210Pb radionuclide from the atmosphere into leaves and stems of plants and soil environment, by the potential soil pollution from around industry zones, therefore by 210Pb uptake of plants from the soil environment.
Besides, sweet potato leaf absorbed more natural radionuclides as 226Ra, 232Th, and their progenies than the other vegetable samples, especially for amaranthus tricolor.
Calculation of the effective dose rate due to ingestion of vegetables (terrestrial food)
The values of EV from different vegetables were calculated based on the formula (4) and given in Table 7.
It can be seen that turnip, jute plant, and sweet potatoes leaf cause rather high internal exposure due to ingestion than the others. The biggest values of the annual effective dose due to ingestion are 0.122 mSv y−1 for turnip. These values for all cases of surveyed vegetables are lower than the world average of 0.290 mSv y−1 (for total ingestion exposure of natural radioactivity) (UNSCEAR, 2000, Table 31, Annex B) [13]. It can be concluded that the radiological impact of surveyed vegetables is negligible to the public health.
Conclusions
-
All the surveyed soil samples after a crop using different fertilizers have the radioactivity concentrations of 238U, 226Ra, 232Th, 40 K, 210Pb varying in the range from 0.5 to 1.5 times compared with the ones in surveyed soil samples before planting. The trend depends on the type of radioisotope and the absorption mechanism of the vegetable. Particularly, the radioactivity concentrations of 238U, 226Ra, and 210Pb in the area using phosphorus fertilizer have increased from 45% (for 226Ra) to 73% (for 238U).
-
The soil samples after harvesting have a reduced equivalent radium activity. During the absorption and development process, the plant has absorbed radioisotopes. Therefore, with a moderate amount of fertilizer, the soil after a crop has almost no radioactive residue from fertilizer, except for the area using phosphorus fertilizer.
-
The equivalent radium activity of all soil samples after harvesting is less than the limit of 370 Bq kg−1. The values of the annual effective dose due to ingestion for all cases of surveyed vegetables are less than the world average of 0.290 mSv y−1. There are no signs of radioactive residues in agricultural land due to fertilizer in the surveyed area after a crop.
-
It can be concluded that the radiological impact of surveyed vegetables is negligible.
References
Khater AEM, Higgy RH, Pimpl M (2001) Radiological impacts of natural radioactivity in Abu-Taror phosphate deposits. Egypt J Environ Radioact 55:255–267
Loan TTH, Ba VN, Dao NQ, Anh NN, Man MT, Thy THN, Hong HTY, Thang NV, Hoang TM (2018) Estimation of soil characteristics based on the depth distributions of 238U, 232Th, 226Ra, 40K activity concentrations using laboratory HPGe gamma spectrometry. J Radioanal Nucl Chem 318:1931–1938
Bolca M, Saç MM, Çokuysal B, Karalı T, Ekdal E (2007) Radioactivity in soils and various foodstuffs from the Gediz River Basin of Turkey. Radiat Meas 42:263–270
Al-Kharouf SJ, Al-Hamarneh IF, Dababneh M (2008) Natural radioactivity, dose assessment and uranium uptake by agricultural crops at Khan Al-Zabeeb, Jordan. J Environ Radioact 99:1192–1199
Asaduzzaman Kh, Khandaker MU, Amin YM, Bradley DA, Mahat RH, Nor RM (2014) Soil-to-root vegetable transfer factors for 226Ra, 232Th, 40K and 88Y in Malaysia. J Environ Radioact 135:120–127
Al-Hamarneh IF, Alkhomashi N, Almasoud FI (2016) Study on the radioactivity and soil-to-plant transfer factor of 226Ra, 234U and 238U radionuclides in irrigated farms from the northwestern Saudi Arabia. J Environ Radioact 160:1–7
Greger M (2004) Uptake of nuclides by plants (SKB-TR-04-14) Sweden
Karunakara N, Rao C, Ujwal P, Yashodhara I, Kumara S, Ravi PM (2013) Soil to rice transfer factors for 226Ra, 228Ra, 210Pb, 40K and 137Cs: a study on rice grown in India. J Environ Radioact 118:80–92
Canberra Industries, Inc. (2004) Genie 2000 version 3.0—Customization tools manual. Canberra Industries, Inc., USA
Ortec Industries, Inc. (2012) Angle 3.2 software of semiconductor detector efficiency calculation, Ortec Industries Inc., USA
Vidmar T (2010) True coincidence summing corrections CCCC, a deterministic code. Jozef Stefan Institute, Ljubljana
ISO (2007), International Standard ISO 18589-3, ISO
UNCEAR (2000) Sources and effects of ionizing radiation, volume I, dose assessment methodologies, United Nations Scientific Committee on the Effects of Atomic Radiation, Report to the general assembly, with scientific annexes, United Nations, New York
Saueia CHR, Mazzilli BP (2006) Distribution of natural radionuclides in the production and use of phosphate fertilizers in Brazil. J Environ Radioact 89:229–239
UNSCEAR (2017) Sources, effects and risks of ionizing radiation, UNSCEAR 2016. Report to the general assemply with scientific annexes. United Nations, New York
ICRP (2012) Compendium of dose coefficients based on ICRP Publication 60. ICRP Publication 119. Annals of ICRP 41 (Suppl. 1). International Commission on Radiological Protection, Elsevier Ltd., Oxford
Ministry of Health, National Institute of Nutrition (2010) General nutrition survey 2009–2010, UNICEF, Medical Publishing House
Loan TTH, Ba VN, Bang NVT, Thy THN, Hong HTY, Huy NQ (2018) Natural radioactivity and radiological health hazard assessment of chemical fertilizers in Vietnam. J Radioanal Nucl Chem 316(1):111–117
IAEA (2013) Radiation protection and mamagement of NORM residues in the phosphate industry. Safety reports series No. 78, IAEA, Vienna
Menzel RG (1965) Soil- plant relationships of radioactive elements. Health Phys 11:1325–1332
Verkhovskaya IN et al (1969) The content and translocation of natural radioactive elements in the system ‘soil-plants-animals’ under natural and experimental conditions. In: Radioecology (Proceedings of International Symposium), Départment de protection sanitaire, CEA, Centre d’études nucléaires de Fontenay-aux-Roses, pp 781–832
Acknowledgements
This work is funded by The National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.04-2019.10. This research is done at the Nuclear Technique Laboratory (NTLab), VNUHCM-University of Science, which was invested by Vietnam National University Ho Chi Minh City, Vietnam.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Loan, T.T.H., Ba, V.N., Dan, D.T.T. et al. Impacts of TENORM from fertilizers on soil and vegetables and the effective dose rate due to ingestion of vegetables at the agricultural zone in Vietnam. J Radioanal Nucl Chem 327, 609–616 (2021). https://doi.org/10.1007/s10967-020-07547-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07547-1