Abstract
Calcium chloride (CaCl2) roasting and nitric acid (HNO3) leaching were combined to remove uranium and radioactivity from uranium tailings. The results show that the uranium and gross α and β radioactivity removal efficiencies improve with increasing CaCl2 addition and HNO3 leaching time and reach maximum values of 81.5%, 87.9% and 86.5%, respectively, after roasting with 50% CaCl2 for 120 min, followed by 1 mol L−1 HNO3 leaching. The uranium leaching rate is 62.3% higher than with direct acid leaching. The mineralogical characteristics of the roasted clinker show that roasting uranium tailings with CaCl2 changes their mineral phase, and Ca2+ becomes incorporated into the silicate matrix, which is beneficial for removing uranium and other radionuclides by acid leaching.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is an important strategic resource [1]. In addition to contributing substantially to the field of nuclear fuel, uranium resources are widely used in scientific research, medicine, industry, national defence and other fields [2, 3]. The rapid development of nuclear technology has gradually increased the demand for uranium mining and smelting products, resulting in a large amount of uranium tailings [1, 4]. It is worth noting that China’s uranium tailings reserves have reached 200 million tons (t) [5]. They contain 0.005–0.02% uranium and a large amount of radionuclides [6, 7]. During the long-term storage of uranium tailings, uranium and radionuclides may be transferred to the natural environment by geological factors such as weathering and rainfall and then enter the human body through the food chain, thus resulting in internal radiation and other harmful effects on human health [8,9,10,11]. The removal of uranium and radioactivity from uranium tailings has attracted widespread concern.
Uranium in tailings is generally stored in gangue minerals, such as quartz, which is difficult to leach by traditional methods [2, 4]. Enormous efforts have been devoted to studying the removal mechanism of uranium from low-grade and refractory uranium tailings. Wang et al. [4] applied a 4854 t plant-scale heap bioleaching process to recover uranium from uranium tailings and found that uranium present in quartz structures was difficult to extract. Li et al. [2] concluded that low-concentration alkaline solutions can selectively destroy the structure of quartz and gangue minerals in uranium tailings to promote the formation of micropores in the particles, increasing the likelihood of contact between the leaching agent and uranium. Huang et al. [12] combined a dilute alkali pretreatment with sulfuric acid leaching to remove uranium from uranium tailings and investigated the effects of the alkali concentration, liquid–solid ratio, and pretreatment time on uranium leaching. The results showed that the leaching efficiency of uranium after the dilute alkali pretreatment was higher than that achieved with direct acid leaching. Zhang et al. [5] concluded that the extraction of uranium from uranium tailings by oxidative leaching with H2O2 promotes the dissolution of the complex gangue structures and enhances the reaction rate between the uranium phases and H2SO4; however, changes in the residue surface cause more uranium to be adsorbed on the surface. Yang et al. [13] pretreated uranium tailings with microwaves, which resulted in a significant increase in the dissociation of uranium monomers enclosed in the gangue structure. These pretreatments improved the external properties of uranium inclusions (for example, by increasing the number of pores and cracks) or destroyed the gangue structure to increase the likelihood of uranium contact with the leaching agent, but none of these methods completely changed the phase composition of the uranium inclusions. Improved pretreatment methods that promote the transformation of uranium inclusions into phases sensitive to leaching agents may be an effective means of uranium resource recovery.
CaCl2 roasting is an excellent method for converting inert components in minerals into substances sensitive to leaching agents. From the perspective of phase transformation, the presence of quartz and Fe2O3 in the target mineral promotes the decomposition of CaCl2 under high-temperature conditions [14]. Then, calcium ions are incorporated into the Si–O–Si structure of the inert quartz and silicate to form new silicates, which weakens the stable structure [15, 16]. Because the strength of the Ca–O bond is lower than that of the Si–O bond [15], the new silicate phase is more easily attacked by the acid leaching agent than the original phase [17]. In addition, CaCl2 roasting is a green and environmentally friendly treatment method [14], and it is highly efficient and inexpensive for extracting refractory metals [18,19,20]. Therefore, it is widely used to treat fly ash [16, 17, 21], potassium feldspar [22], kaolin [23], and cyanide tailings [14] to obtain target metals. Sun et al. [17] studied the removal of uranium and radioactivity from fly ash using magnetic separation, mechanical activation combined with alkaline activation, and CaCl2 roasting followed by HNO3 leaching. The results showed that the uranium leaching rate was 95.8% under the optimal CaCl2 roasting conditions and the main location of uranium changed from the vitreous materials to the newly formed calcium silicate. Li et al. [14] concluded that silica was converted to a series of silicates after CaCl2 calcination during the extraction of gold and silver from cyanide tailings. Yuan et al. [22] utilized CaCl2 calcination to extract potassium from potassium feldspar and observed new phases, such as wollastonite, pseudowollastonite, and anorthite, in the roasted product. CaCl2 plays a key role in mineral phase transformation during CaCl2 calcination. In addition, the acid decomposition of the silicate formed in the roasted clinker provides a theoretical basis for recovering the target metal [15, 24]. Nevertheless, attempts to remove uranium from uranium tailings using CaCl2 roasting have not been reported.
In this study, CaCl2 roasting was combined with HNO3 to remove uranium and radioactivity from uranium tailings. The effects of the CaCl2 addition ratio and HNO3 concentration on the removal of uranium and radioactivity were investigated. The minerals around uranium before and after calcination were qualitatively analysed by electron probe microanalysis (EPMA) technology, and the possible removal mechanism of uranium and radioactivity was initially explored by investigating the phase transformation of the minerals and the storage location of uranium. The research results provide a scientific basis and theoretical support for the removal of uranium and radioactivity from uranium tailings.
Experiments
Materials
The samples used in this study were obtained from a uranium tailings depot in Jiangxi Province, China. The uranium tailings were dried to a constant weight in an electric blast drying oven (DHG-9070A, Shanghai Huitai Instrument Manufacturing Co., Ltd., China) at 60 °C for 24 h. After natural cooling, impurities such as plant roots were removed from the sample, and the resulting samples were ground to a powder using a planetary ball mill (QM-3SP2, Shanghai Optical Instrument Factory, China) and passed through a 100-mesh standard sieve. The samples were thoroughly mixed, and a small amount was removed for chemical component analysis (Table 1) by X-ray fluorescence (XRF) spectrometry and radioactivity analysis (gross α of 14.30 Bq/g and gross β of 15.00 Bq/g). The remaining samples were stored in a silica desiccator until use during the experiments. The samples consisted of uranium tailings produced by a series of processes (acid leaching) and containing a low-grade form of uranium that is difficult to treat.
Direct HNO3 leaching
A total of 10.00 g of a powder sample of uranium tailings and 100 mL of an HNO3 solution (1 mol L−1, 2 mol L−1, 4 mol L−1, or 8 mol L−1) were added to a 500 mL Erlenmeyer flask, which was then sealed and placed in a water bath thermostat (ZWF-110X50, Shanghai Zhicheng Analytical Instrument Manufacturing Co., Ltd., China). Direct HNO3 leaching was performed at 80 °C and 150 rpm to keep the phase interface in constant flux, ensuring uniform mixing of the solid and liquid phases in the leaching process. During 5-, 10-, 15-, 30-, 60- and 120-min leaching processes, 1.5 mL of the supernatant was filtered through a 0.22 μm filter to measure the uranium concentration. After soaking for 240 min, solid–liquid separation was conducted by centrifugation at 10,000 rpm for 10 min in a centrifuge (Thermo Sorvall Lynx 6000), and the supernatant was collected. The solid residue was rinsed with water until no ions were detected and then dried at 105 °C for 24 h, and the weight was recorded. Each leaching experiment was performed in parallel.
CaCl2 roasting combined with HNO3 leaching
Accurately weighed CaCl2 (1.00 g (10%), 2.00 g (20%), 4.00 g (40%), 5.00 g (50%), or 10.00 g (100%)) was passed through a 100-mesh standard sieve after grinding with an agate mortar and then thoroughly mixed with 10.00 g of uranium tailings. The mixture was placed in a corundum crucible and baked in a muffle furnace (XY-MF-5-12, Shanghai Xinyi Instrument Co., Ltd., China). The roasting process was as follows: the muffle furnace was heated from room temperature (RT, 25 °C) to 900 °C at a heating rate of 10 °C/min and then held at 900 °C for 120 min. After heating, the sample was cooled to RT. The clinker was weighed, and the value was recorded to calculate the weight loss. An appropriate amount of clinker was used for characterization analyses, and the rest of the clinker was ground to less than 100 mesh, washed with deionized water three times, dried, and then stored in a silica desiccator. Roasted clinker with different addition ratios of CaCl2 was mixed with 100 mL of HNO3 (0.01 mol L−1, 0.1 mol L−1, 1 mol L−1, 4 mol L−1, or 8 mol L−1) in an Erlenmeyer flask, and the flasks were placed in a constant-temperature water bath shaker at RT and 150 rpm. The process, including the sampling time and method, the end of the leaching treatment and the determination of the uranium concentration, was the same as that for direct HNO3 leaching. Each experiment was performed in duplicate, and the slag was dried for 24 h before detecting the radioactivity and performing characterization analyses.
Analytical methods and characterization
The supernatant was centrifuged, filtered through a 0.22 μm filter, diluted and acidified to pH < 2.0, and the uranium concentration was measured by inductively coupled plasma emission spectrometry (ICP-OES, Agilent 5100, Agilent Technologies (China) Co., Ltd.). The ICP-OES operation steps were as follows: the measurement wavelength was λ = 409.01 nm, and after the plasma was ignited, the pipeline, atomizer and atomization chamber were cleaned with 5% HNO3. A standard curve was prepared by analysing uranium standard samples, and a correlation coefficient greater than 0.9999 was obtained. This curve was used to determine the uranium concentrations of the samples.
Scanning electron microscopy-energy dispersive spectroscopy (SEM–EDS, Nova Nano SEM450 US FEI) was used to analyse the surface morphology and elemental composition changes of the raw ore samples, direct HNO3 leaching residue, and 100% CaCl2-roasted clinker. The ore samples were randomly mixed and gold-plated with an automatic ion-sputtering coater. An appropriate position and magnification were selected to observe the morphology of each sample, and EDS spectral analysis was performed.
The washed and dried ore samples were ground to pass through a 200-mesh standard sieve, and then polycrystalline X-ray diffraction (XRD, D8-A25, BRUKER, Germany) was used to analyse the mineral phase composition and determine the crystal structure and composition. The mineral phases were qualitatively analysed by comparing the obtained spectral lines of the mineral crystal structure with standard PDF cards. The X-ray tube voltage was 40 kV, the tube current was 40 mA, the scanning angle 2θ ranged from 10° to 80° with an increment of 0.02046°, and a scanning time of 0.150 s was used.
The samples were studied using an electron probe microanalyser (EPMA, JXA-8100, Jeol Ltd., Japan). Electron probe X-ray microanalysis is a method for qualitatively and quantitatively analysing inorganic solid microregions and studying their microstructure and morphological characteristics. Microareas of the ore samples were studied by single-point elemental analysis to determine the differences in the compositions of the areas surrounding uranium before and after roasting and thus the state of uranium. The samples were plated with carbon and fixed to the sample stage in the instrument, which was operated at 15 kV and 10−7 A.
After leaching, the slag was washed and dried, and the gross α and β radioactivity values of the solid samples were measured at the Radiation Environment Monitoring Center of the Guangdong Province Nuclear Industry Geological Bureau. A multi-channel low-background α/β detector (PAB-6000IV) was used to determine these values in the leached slag samples according to “Determination of gross alpha activity in water: thick source method” (EJ/T1075-1998 [25]) and “Determination of gross beta activity in water: evaporation method” (EJ/T900-94 [26]).
Results and discussion
Effects of CaCl2 roasting on uranium extraction from uranium tailings
The uranium leaching rate of the uranium tailings is shown in Fig. 1. When the HNO3 concentration was increased from 1 to 8 mol L−1 in the direct HNO3 direct leaching process, the uranium leaching rate did not improve significantly, only increasing from 19.2 to 26.2%. These results indicate that only a small proportion of the total uranium, possibly the exchangeable, carbonate-bound, Fe–Mn oxide-bound, and organic matter-bound fractions, could be released by HNO3 leaching, whereas the majority of the uranium remained in the residual fraction [11, 27, 28]. After CaCl2 roasting, the leaching rate of uranium increased with increasing HNO3 concentration. When the HNO3 concentration was 8.0 mol L−1, 88.2% of the uranium was released into the acid solution, showing that roasting uranium tailings with CaCl2 improved the extractability of the inert silicate and uranium components. In addition, the leaching temperature used for the CaCl2-roasted clinker was significantly lower than that required for the sample obtained by direct HNO3 leaching.
Effects of the CaCl2 addition ratio and HNO3 leaching time on the uranium extraction efficiency
Figure 2 presents the effects of CaCl2 addition and the HNO3 leaching time on the uranium extraction efficiency. As shown in Fig. 2a, the leaching rate of uranium increased linearly from 26.3 to 81.5% as the addition of CaCl2 increased from 10 to 50%, and then it remained nearly unchanged, that is, when the addition ratio of CaCl2 increased from 50 to 100%, the leaching rate of uranium increased by only 1.6%. These results indicate that increasing the addition ratio of CaCl2 within a certain range can enhance the reaction between uranium tailings and CaCl2, which further activates the crystal structure in the uranium tailings and thus improves the leaching of uranium. The trend in the leaching rate of the uranium tailings obtained at different leaching times 1.0 mol L−1 HNO3 after CaCl2 roasting was similar to that obtained as a function of the proportion of CaCl2, that is, the uranium leaching rate increased rapidly and then remained unchanged (Fig. 2b). Most of the uranium (81.5%) was rapidly leached in 120 min, indicating that the uranium tailings released more than half of the uranium in the mineral matrix (probably silicate) after CaCl2 roasting. Within the next 200 min, the leaching rate of uranium increased by only 0.5%. In terms of energy consumption, a leaching time of 120 min was the optimal time tested.
Removal of gross α and β from uranium tailings
The variations in the removal rate and specific activities of gross α and β at different HNO3 concentrations are shown in Fig. 3a. At 50% CaCl2 addition, the gross α removal rate increased sharply from 42.5 to 87.9% as the HNO3 concentration increased from 0.01 to 1 mol L−1, and the gross β removal rate increased from 51.5 to 86.5%. At higher HNO3 concentrations, the removal of gross α and β was no longer sensitive to changes in the concentration. Overall, the gross α and β removal rates increased with increasing HNO3 concentration, but those obtained by leaching with 0.1 mol L−1 HNO3 were the lowest. This result might be due to a slight increase in the acidity and the acid etching of crystalline phases (such as anorthite) in the roasted product with the dissolution of silica. The presence of fluoride ions (Table 1) at lower pHs accelerates the polymerization of monomeric silica and increases the amount of precipitates in the pores of the mineral [24]. This process inhibits the transfer of radionuclides into solution. In addition, it is possible that precipitates formed by metals such as Al and Ca reabsorb the leached uranium [29].
As the addition ratio of CaCl2 increased from 10 to 50%, the gross α and β removal rates increased from 14.8 to 87.9% and from 31.4 to 86.5%, respectively (Fig. 3b). When the addition ratio of CaCl2 was increased from 50 to 100%, the removal rates of gross α and β decreased slightly. The slight decrease in the total amount of radioactivity removed might be attributed to the generation of a large amount of “gelatinized silicate” by the excessive reaction of CaCl2 with quartz, which would result in a high concentration of dissolved silica and extensive gelation of the acidic leachate [15, 16]. The adhesion of these “gelatinizing silicates” and amorphous silica gel to the surfaces of the samples prevents the transfer of uranium and other radionuclides into solution. Compared with the results of direct leaching with HNO3, the gross α and β removal rates decreased when 10% CaCl2 was added, which might be due to the incomplete reaction between CaCl2 and the sufficient silicate in the uranium tailings. The remaining inert and stable materials in uranium tailings, such as quartz and feldspar, can significantly enhance the diffusion of ion radioisotopes from the initially contaminated surface to the mineral matrix after calcination at high temperatures, reducing their leaching rate in solution [16, 30].
Mineralogical transformation and morphological changes in the uranium tailings
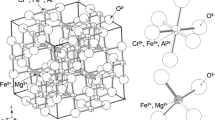
Figure 4 shows the XRD patterns of the uranium tailings before and after direct HNO 3 leaching and of the CaCl2-roasted clinker. The main mineral phase in the original uranium tailings slag was quartz, and the minor phases included orthoclase, soda feldspar, muscovite, and a small amount of illite. Illite may be produced by muscovite weathering in uranium tailings, and radionuclides may migrate through weathering. Compared with the diffraction pattern of the original uranium tailings, the diffraction peaks of orthoclase and illite disappeared after direct leaching with 4 mol L−1 HNO3 for 2 h at 80 °C, which could be due to HNO3 erosion. However, the effects of HNO3 cannot successfully destroy stable mineral phases such as quartz and sodalite. After calcination of the CaCl2 and uranium tailings mixture, the silica in the original uranium tailings was destroyed, and new phases, such as wollastonite, calcareous feldspar, and apatite, appeared, indicating that the stable quartz crystals were transformed into a series of silicates. Lei et al. [16] and Liang et al. [21] noted the absence of a quartz phase in the XRD diffraction pattern of roasted clinker (fly ash and CaCl2), and they achieved a removal rate of the target metal of over 90%. However, in this study, a small amount of the quartz phase remained, and a new phase, moganite, was generated, which might explain the poor removal of uranium and radioactivity.
Figure 5a shows that the original surface of the uranium tailings was smooth, with a small amount of debris and a few cracks. As shown in Fig. 5b, after leaching with 1 mol L−1 HNO3, part of the ore surface became rough, which might have been caused by surface erosion by HNO3 and cracks in the ore sample. However, the surfaces of some of the minerals remained smooth, possibly because HNO3 cannot easily attack the stable materials in uranium tailings. Because the 900 °C roasting temperature is between the melting point (782 °C) and boiling point (1600 °C) of CaCl2, CaCl2 reacts with the uranium tailings in both the molten and solid states [31]. As shown in Fig. 5c, for the roasted clinker with 100% CaCl2 addition, the uranium tailings reacted fully with the two states of CaCl2, resulting in an improved mineralogical transformation. Based on both the XRD analysis and EDS data (Fig. 5d), CaCl2 can effectively change the structure and composition of uranium tailings through the roasting activation pathway. The presence of SiO2 in the minerals can promote the decomposition of calcium chloride [31], and calcium ions enter the Si–O–Si structure to form new silicates [16]. The newly formed phases are more susceptible to HNO3 dissolution and erosion than stable silica [15].
CaCl2 roasting combined with HNO3 leaching to remove uranium and total radioactivity
Our research team previously used the Tessier method for the continuous and stepwise extraction of uranium to obtain its main distribution in uranium tailings: the exchangeable, carbonate-bound, organic matter-bound, Fe–Mn oxide-bound, and residual fractions accounted for 0.87%, 1.22%, 15.74%, 8.58%, and 73.59%, respectively [11]. The proportion of silica (65.76%) in the chemical composition of uranium tailings minerals (Table 1) is similar to the proportion of uranium in the residual fraction (73.59%) obtained by Wang et al. [11] using the Tessier continuous extraction method. In addition, the main diffraction peak in the XRD pattern of the uranium tailings was attributed to quartz (Fig. 4), and the surrounding minerals were determined to be mainly quartz with a small amount of orthoclase and albite by EPMA (Fig. 6), which indicates that uranium and radionuclides mainly coexist with silica in uranium tailings. This result is consistent with those of Zhou et al. [1] and Wang et al. [4], who found that the refractory uranium in uranium tailings after microbial leaching is encapsulated in quartz particles. Therefore, it is difficult to remove uranium and other radionuclides from uranium tailings because uranium and its daughter radionuclides are either incorporated or entrapped in the silica matrix. After leaching with 4 mol L−1 HNO3 at 80 °C for 2 h, the recovery rate of uranium was only 25.6%, indicating that HNO3 cannot easily destroy the stable silica structure, which prevents the transfer of uranium and radionuclides into the solution and thus results in their unsatisfactory removal.
After the uranium tailings were roasted with CaCl2, the silica and surrounding rock minerals in the ore were converted to wollastonite, silicate and other new phases according to formulas (1)–(4) (Figs. 5, 6). Because the strengths of the metal cation-oxygen bonds in the silicates are lower than that of silicon-oxygen bonds, the former is more susceptible to acid attack, and the reactions in formulas (5)–(7) occur [15]. At the same time, the good binding capacity of calcium enables the molten CaCl2 to become more reactive during roasting, and Ca2+ might migrate into the silicate matrix [32], resulting in the complex transformation of the mineral components and thus an increase in the susceptibility of the roasted uranium tailings to acid attack. The reactions with acid probably occur according to formulas (8)–(10).
When silicates undergo acid decomposition, the uranium and other radionuclides in them or mixed with them are simultaneously leached by acid from the solid tailings into the aqueous solution. However, it is worth noting that due to the dissolution of the metal cations and silica, the silicate structure is completely destroyed, and the silica dissolved in the solution easily polymerizes to form silica gel [15]. The formation of silica gel hinders the leaching of uranium and other radionuclides. Therefore, the proportion of CaCl2 added is a key parameter that must be considered.
Environmental benefits of removing uranium and radioactivity from uranium tailings
These experimental results confirm that removing uranium and radioactivity from uranium tailings is feasible. First, low-grade and refractory uranium tailings are an important way to obtain uranium because these abundant resources are stored in a large number of uranium tailings depots. Second, the removal of uranium and radioactivity from uranium tailings can effectively reduce their threat to the environment and also decrease the uranium tailings heap area during the treatment process. Third, the CaCl2 roasting process is a highly efficient and low cost method for extracting refractory metals that can be easily increased to industrial scale. Finally, alumina and ferric oxide can be recovered during the process.
Conclusions
In this work, CaCl2 roasting was combined with nitric acid leaching to remove uranium and radioactivity from uranium tailings. The results show that uranium and gross radioactivity can be effectively removed from uranium tailings by CaCl2 roasting combined with nitric acid leaching. Compared with direct acid leaching (19.2–26.2%), the leaching rate of uranium increased significantly to 83.1–88.2% when the uranium tailings were calcined with 100% CaCl2 for 2 h at 900 °C and then leached with 1–8 mol L−1 HNO3 for 2 h. As the amount of CaCl2 added was increased from 10 to 50% and the HNO3 leaching time was lengthened from 5 min to 1 h, the removal efficiencies of uranium and gross α and β radioactivity increased dramatically. However, when the dosage of CaCl2 was further increased or the acid leaching time was prolonged, no additional gains in the efficiency were achieved. After roasting the uranium tailings with 50% CaCl2, most of the uranium (81.5%) and gross α (87.9%) and β (86.5%) radioactivity could be removed by 1 mol L−1 HNO3 in 120 min. The mineralogical characteristics of the roasted clinker show that the calcined CaCl2 could change the mineral phases of the uranium tailings. Molten CaCl2 promotes Ca2+ incorporation into the silicate matrix of uranium tailings, which gradually makes them susceptible to acid attack. The newly formed silicate is decomposed by acid, and uranium and other radionuclides are leached from the roasted clinker by the acid. The research results demonstrate very good application prospects for recovering uranium and removing radioactivity from uranium tailings.
References
Zhou Z, Yang Z, Sun Z, Chen G, Xu L, Liao Q (2019) Optimization of bioleaching high-fluorine and low-sulfur uranium ore by response surface method. J Radioanal Nucl Chem 322(2):781–790
Li M, Huang C-M, Zhang X-W, Gao F-Y, Wu X-Y, Fang Q, Tan W-F, Zhang D (2018) Extraction mechanism of depleted uranium exposure by dilute alkali pretreatment combined with acid leaching. Hydrometallurgy 180:201–209
Singh DK, Hareendran KN, Sreenivas T, Kain V, Dey GK (2017) Development of a phosphate precipitation method for the recovery of uranium from lean tenor alkaline leach liquor. Hydrometallurgy 171:228–235
Wang X, Liu Y, Sun Z, Li J, Chai L, Min X, Guo Y, Li P, Zhou Z (2017) Heap bioleaching of uranium from low-grade granite-type ore by mixed acidophilic microbes. J Radioanal Nucl Chem 314(1):251–258
Zhang B, Li M, Zhang X, Huang J (2016) Kinetics of uranium extraction from uranium tailings by oxidative leaching. JOM 68(7):1990–2001
Akhtar S, Yang X, Pirajno F (2017) Sandstone type uranium deposits in the Ordos Basin, northwest China: a case study and an overview. J Asian Earth Sci 146:367–382
Yan X (2015) Migration and distribution of radionuclide in uranium tailing soil and the effects of radionuclideon soil microbial diversity. Dissertation, Northeast Forestry University
Liu B, Peng T, Sun H (2017) Leaching behavior of U, Mn, Sr, and Pb from different particle-size fractions of uranium mill tailings. Environ Sci Pollut Res 24:15804–15815
Yan X, Luo X (2015) Radionuclides distribution, properties, and microbial diversity of soils in uranium mill tailings from southeastern China. J Environ Radioact 139:85–90
Zheng L, Zhou Z, Rao M, Sun Z (2020) Assessment of heavy metals and arsenic pollution in surface sediments from rivers around a uranium mining area in East China. Environ Geochem Health 42(5):1401–1413
Wang Y (2017) Characteristics of soils contaminated with uranium in uranium mining area and experimental study on remediation by srb dominant mixed bacteria. Dissertation, East China University of Technology
Huang C, Li M, Zhang X, Gao F, Wu X, Fang Q, Tan W, Zhang D (2018) Uranium extraction from tailings by dilute alkali pretreatment-sulfuric acid leaching technology. JOM 70(11):2746–2752
Yang Y, Yu Q, Hu N, Ding D (2016) Effect of microwave pretreatment on leaching behavior of uranium in heap-leached uranium tailings and its mechanism. Chin J Nonferrous Met 26(6):1356–1363
Li H, Ma A, Srinivasakannan C, Zhang L, Li S, Yin S (2018) Investigation on the recovery of gold and silver from cyanide tailings using chlorination roasting process. J Alloys Compd 763:241–249
Terry B (1983) The acid decomposition of silicate minerals part I. Reactivities and modes of dissolution of silicates. Hydrometallurgy 10 (2):135-150
Lei X, Qi G, Sun Y, Xu H, Wang Y (2014) Removal of uranium and gross radioactivity from coal bottom ash by CaCl2 roasting followed by HNO3 leaching. J Hazard Mater 276:346–352
Sun Y, Qi G, Lei X, Xu H, Wang Y (2016) Extraction of uranium in bottom ash derived from high-germanium coals. Procedia Environ Sci 31:589–597
Yan Q, Li X, Wang Z, Wang J, Guo H, Hu Q, Peng W, Wu X (2012) Extraction of lithium from lepidolite using chlorination roasting–water leaching process. Trans Nonferr Metal Soc China 22(7):1753–1759
Ojeda MW, Perino E, Ruiz MdC (2009) Gold extraction by chlorination using a pyrometallurgical process. Miner Eng 22(4):409–411
Brocchi EA, Moura FJ (2008) Chlorination methods applied to recover refractory metals from tin slags. Miner Eng 21(2):150–156
Liang Z, Lei X, Sun Y, Xu H, Wang Y (2013) Extraction of alumina from coal fly ash by calcination with calcium chloride process. China Environ Sci 33(9):1601–1606
Yuan B, Li C, Liang B, Lü L, Yue H, Sheng H, Ye L, Xie H (2015) Extraction of potassium from K-feldspar via the CaCl2 calcination route. Chin J Chem Eng 23(9):1557–1564
Zheng S, Li P, Tian L, Cao Z, Zhang T, Chen Y, Zhang Y (2016) A chlorination roasting process to extract rubidium from distinctive kaolin ore with alternative chlorinating reagent. Int J Miner Process 157:21–27
Terry B (1983) The acid decomposition of silicate minerals part II. Hydrometallurgical applications. Hydrometallurgy 10(2):151–171
EJ/T1075-1998 (1998) Determination of gross alpha activity in water thick source method. Nuclear industry standard of the People’s Republic of China
EJ/T900-94 (1995) Determination of gross beta radioactivity in water evaporation method. Nuclear industry standard of the People’s Republic of China
Liang L, Feng Z, Ma Q, Zhang B, Li S (2014) Study on the occurrence form and activity of uranium in Hunan uranium tailings. Environ Pollut Control China 02:11–14
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–850
Szecsody JE, Truex MJ, Qafoku NP, Wellman DM, Resch T, Zhong L (2013) Influence of acidic and alkaline waste solution properties on uranium migration in subsurface sediments. J Contam Hydrol 151:155–175
Spalding B (2001) Fixation of radionuclides in soil and minerals by heating. Environ Sci Technol 35:4327–4333
Liu J, Wen S, Chen Y, Liu D, Bai S, Wu D (2013) Process optimization and reaction mechanism of removing copper from an Fe-rich pyrite cinder using chlorination roasting. J Iron Steel Res Int 20(8):20–26
Baoguo Ma, Meng Qi, Jun Peng, Zongjin L (1999) The compositions, surface texture, absorption, and binding properties of fly ash in China. Environ Int 25:423–432
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (41662024), the Natural Science Foundation of Jiangxi Province (20142BAB213021) and the independent fund of the State Key Laboratory of Nuclear Resources and Environment of China (Z1917).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yubo, G., Zhongkui, Z., Jiamin, L. et al. Combined use of CaCl2 roasting and nitric acid leaching for the removal of uranium and radioactivity from uranium tailings. J Radioanal Nucl Chem 325, 657–665 (2020). https://doi.org/10.1007/s10967-020-07272-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07272-9