Abstract
A highly-enriched 244Pu isotope dilution reference material has been prepared and characterized for metrologically traceable measurements of very small quantities of plutonium. The amount of plutonium in samples associated with nuclear safeguards and nuclear forensic measurements can be significantly less than 1 ng. Accordingly, the ability to quantify the amount and isotopic composition of plutonium from a single mass-spectrometric analysis is particularly desirable. The highly-enriched 244Pu reference material, described here, will minimize the magnitude of spike corrections necessary to obtain accurate information on plutonium isotopic composition from isotope dilution measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Highly-enriched 244Pu makes an ideal isotopic spike for plutonium amount measurements by isotope dilution mass spectrometry (IDMS). The 244Pu nuclide has the longest half-life of the plutonium isotopes (8.0 × 107 a [1]) and occurs at very low abundance in nature [2] or as fallout from nuclear testing [3]. Similarly, plutonium produced in commercial, research, or weapons production reactors has little to no 244Pu. This is due to the necessity for abundant 242Pu, long irradiation times, and high neutron fluxes to generate the 244Pu nuclide in proportions that are significant relative to other plutonium isotopes [4].
To date, there have been two 244Pu certified reference materials available to the analytical community for IDMS measurements, Certified Reference Material (CRM) 131 [5] and IRMM-042a [6]. CRM 131 can be obtained from the NBL Program Office of United States Department of Energy (US DOE) and is comprised of approximately 1 mg of plutonium enriched to a 244Pu atom fraction of 0.97895 n(244Pu)/n(Pu). A unit of IRMM-042a is a nitric acid solution containing 1 µg of 0.9793 n(244Pu)/n(Pu) plutonium that is available from the European Commission’s Joint Research Center, Geel. While these reference materials are suitable for plutonium amount measurements, the relatively high abundances of other plutonium isotopes (e.g. > 0.013 n(242Pu)/n(Pu) for both materials) limits the utility of these CRMs for some nuclear safeguards and nuclear forensic measurement such as environmental samples taken as part of a nuclear safeguards inspection or interdicted uranium materials analyzed for plutonium content and isotopic composition. The absolute amount of plutonium in these types of samples is likely to be minute (frequently less than 1 ng) and can vary from sample to sample by an order of magnitude or more [7]. Adding an enriched 244Pu isotopic spike to a sample during preparation will allow a single mass spectrometric measurement to provide information on the amount of plutonium and the plutonium isotopic composition. Thus, eliminating the need to split a sample prior to spiking in order to perform a separate isotopic composition analysis. There is, however, the potential to inadvertently over-spike small and highly variable safeguards or nuclear forensic samples. If a 244Pu spike has a significant proportion of other plutonium isotopes, the correction applied to over-spiked samples will result in large uncertainties for the isotopic composition.
To assure that plutonium safeguards measurements can be made with a high degree of accuracy and precision, the International Atomic Energy Agency (IAEA) initiated a project to produce a plutonium isotopic tracer with a 244Pu enrichment of greater than 0.999 n(244Pu)/n(Pu) [8]. For this project, the US DOE provided the IAEA with 0.5 g of “FP-33″ plutonium that had a 244Pu atom fraction of approximately 0.17 n(244Pu)/n(Pu). The IAEA worked closely with the Russian Research Institute of Experimental Physics (VNIIEF) where two rounds of electro-magnetic separation were performed on the FP-33 material to prepare a small quantity of plutonium (slightly less than 900 µg) enriched to greater than 0.99 n(244Pu)/n(Pu) [9]. This plutonium was then transferred to Lawrence Livermore National Laboratory (LLNL) for production of a new reference material.
The production project for the 244Pu reference material (referred to here as 244Pu Spike) was planned by LLNL in collaboration with staff at the United States National Institute for Standards and Technology (NIST). LLNL also performed the initial analyses, purified the starting material, created the reference material units, and prepared characterization analysis samples. Mass spectrometry measurements for plutonium assay and isotopic composition were then performed by LLNL, by the US DOE Los Alamos National Laboratory (LANL), and by the Commissariat à l’énergie atomique et aux énergies alternatives (CEA) in France. This publication provides the measured attribute values for the 244Pu Spike; a description of preparation, production, and characterization activities; and a discussion of the metrological traceability and uncertainty evaluation for the attribute values.
Reference material production
This project was planned and executed to assure that the 244Pu Spike reference material would be fit-for-purpose by minimizing the potential for unit-to-unit variability and contamination of the highly enriched starting material. One potential source for unwanted variability is differential contamination resulting in a heterogeneous plutonium isotopic composition for the units. Plutonium in the environment is primarily anthropogenic fallout from atmospheric nuclear weapons testing and occurs at very low concentrations [10]. The LLNL facility where the reference material was prepared has a history of plutonium handling and analysis. Accordingly, there is potential for a slightly elevated plutonium background that could result in differential contamination of the 244Pu Spike. To minimize this possibility, project activities were performed in a dedicated low-contamination work space that was specifically refurbished for this project; all labware and production equipment was cleaned for ultra-trace low-contamination work; and only high-purity reagents (sub-boiling distilled acids, deionized water, etc.) were used for project activities. Area swipes were taken and analyzed to quantify plutonium background in the laboratory work space and plutonium processing blanks for separation chemistry and instrumental analyses were performed regularly. These measurements indicated plutonium background that was below detection limits (critical level for 239Pu, Lc, was 2 fg for the lab swipes) or at levels too low to alter the reference material within the resolution of measurements made for the project.
The highly enriched 244Pu starting material for the project was received at LLNL in two volumetric flasks labelled FP-33-2-A and FP-33-2-B (Fig. 1). A residue of unknown material was observed in the sample containers during an initial examination, so it was determined that a careful purification of the plutonium would be necessary. Preliminary isotopic analyses of plutonium in the two flasks indicated that the materials were isotopically identical, therefore the contents were combined into a single solution and prepared for purification. The combined plutonium was dissolved in 2 mL of 9 mol L−1 HCl (Seastar Chemicals, Inc., British Columbia, Canada)Footnote 1 with 15 µL of concentrated HNO3 added to the solution (Seastar Chemicals, Inc., Canada). This plutonium solution was loaded onto a PolyPrep column (Bio-Rad, California, USA) that was previously prepared with a 1.8 mL resin bed of BioRad AG1x8 (100-200 mesh) anion exchange resin. The column was rinsed with 9 mL of 9 mol L−1 HCl to remove elements not adsorbed onto the resin. Then the plutonium was eluted in 19 mL of a 12:1 mixture of 9 mol L−1 HCl and distilled HI (Sigma-Aldrich, Massachusetts, USA). Finally, the column was stripped using 25 mL of a mixed solution of 0.1 mol L−1 HCL and 0.01 mol L−1 HF (Seastar Chemicals, Inc., Canada) to remove U, Fe, and any small fraction of remaining Pu. The three collected solutions were converted to nitrate and analyzed by inductively couple plasma mass spectrometry (ICP-MS) to determine the efficiency of the plutonium recovery and to verify that the plutonium isotopic composition had not been altered. These analyses indicated that 99.97% of the plutonium was recovered and that any plutonium contamination introduced to the material during the purification processing had not resulted in a resolvable change to the 244Pu isotopic composition.
The purified plutonium was dried and then prepared as a relatively concentrated solution by dissolving the plutonium using 10 mL of a mixed solution comprised of 4 mol L−1 HNO3 and 0.01 mol L−1 HF. Another 10 mL of the 4 mol L−1 HNO3 and 0.01 mol L−1 HF solution was added to the container and a semi-quantitative measurement of plutonium concentration was performed by ICP-MS. An aliquot of the concentrated solution, equivalent to 25 µg to 30 µg of Pu, was transferred to a 1 L fluorinated ethylene propylene (FEP) bottle for preparation of the 244Pu reference material “stock” solution. The aliquot was diluted with 1 L of a mixed 4 mol L−1 HNO3 and 0.05 mol L−1 HF solution to a plutonium concentration of approximately 20 ng g−1. The stock bottle was then capped and hand shaken for 1 min to homogenize the solution. Shaking was repeated multiple times over the course of several weeks prior to dispensing of the solution to unit containers.
Before dispensing the stock solution, unit containers (30 mL FEP bottles) were cleaned for ultra-low contamination clean chemistry by washing the containers with Citranox detergent (Alconox Inc., New York, USA), rinsing with deionized water, and fluxing on a 120 °C hotplate with a mixed solution of 1 mol L−1 HNO3 and 0.05 mol L−1 HF for 24 h. The containers were then emptied, allowed to dry in a HEPA-filtered laminar flow hood, labelled with numbers corresponding to the fill order, and weighed on a calibrated XPE105 electronic balance (Mettler-Toledo, LLC, Ohio, USA). The 244Pu Spike reference material units were produced by aliquoting the stock solution using a Hamilton MicroLab 600 dispenser (Hamilton Company, Nevada, USA). The dispenser was outfitted with a new 5 mL dispensing syringe and new product transfer tubing (i.e., intake and dispensing tubing). Before the 244Pu stock solution was dispensed, 50 mL of 4 mol L−1 HNO3 and 0.05 mol L−1 HF solution was run through the apparatus (10 dispensing cycles) then the uptake tubing was transferred to the stock solution and 20 mL of solution was dispensed prior to filling the first reference material unit. A total of 190 aliquots of the stock solution (5 mL each) were then dispensed to the unit containers during a single work session (≈ 3 h). After filling, the reference material units were capped and reweighed. The average dispensed mass of solution for the production run was (5.6217 ± 0.0024) g (k = 2) with a standard deviation of only 0.0008 g and a maximum range in solution mass of 0.0037 g. Two 15 mL aliquots of the stock solution were dispensed between units #90 and #91. These larger aliquots, referred to as samples “A” and “B”, were prepared as characterization samples to assess laboratory-to-laboratory measurement variability.
The reference material units were prepared for packaging by adding approximately 0.025 mL of a 1 mol L−1 H3PO4 solution (Merck KGaA, Darmstadt, Germany) to each unit as a fixative for the plutonium in the unit bottle. The solution in the units was then dried in a heating block (set to a temperature of 120 °C) within a Class-100 HEPA filtered exhausting fume hood. After drying, the plutonium is entrained in a viscous film formed from the phosphoric acid at the bottom of the unit bottle. Once the units had cooled to room temperature, they were capped and sealed by wrapping the top of the bottle with polytetrafluoroethylene (PTFE) tape which, in turn, was wrapped with vinyl tape to prevent loosening of the cap or unravelling of the PTFE tape. Sealed unit bottles were then individually packaged in heat-sealed sleeves.
Characterization analysis
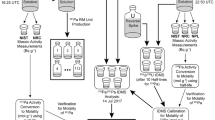
A schematic of the characterization and verification scheme for this project is illustrated in Fig. 2. The main components of the analysis scheme are IDMS measurements of the molality of 244Pu in the stock solution (mol g−1), as dispensed to the sample units, and measurement of the plutonium isotopic composition of the reference material by multi-collector mass spectrometry.
Schematic of 244Pu Spike unit production and characterization process. Arrows in the schematic indicate the transfer of material. Heavy solid lines indicate transfer of 244Pu solution. Double lines indicate direct dispensing of the stock solution and lighter solid lines indicating transfer of reverse-spike solutions
Molality of Pu in the stock solution
The molality of plutonium in the stock solution was measured on randomly selected reference material units. Measurements were made using 3 different IDMS reverse-spikes (Table 1) including a solution prepared from a plutonium metal assay and isotopic reference material, CRM 126-A [11], which was used for the primary molality measurements. Verification IDMS measurements were made in parallel with the primary measurements using a plutonium assay and isotopic standard solution, IRMM 086 [12]. Following these measurements (approximately 6 months after initial IDMS measurements), a third set of IDMS analyses were made using a highly enriched 242Pu isotopic spike, CRM 130 [13]. These later measurements were made to assess an apparent bias between values for molality of plutonium indicated by the primary and verification measurements.
A 0.25 g piece of CRM 126-A Pu Assay and Isotopic Standard with a 239Pu atom fraction of 0.94 n(239Pu)/n(Pu) was prepared for quantitative dissolution in accordance with the CRM certificate recommendations and dissolved in a nitric acid solution. Two quantitative dilutions were performed at LANL before a 20 g aliquot of the second dilution was packaged and shipped to LLNL. A single quantitative dilution using a solution of 4 mol L−1 HNO3 and 0.05 mol L−1 HF (CRM 126-A Dilution 1) was performed at LLNL to obtain a reverse-spike solution with a plutonium concentration in the same range as the 244Pu Spike stock solution. All masses for preparation of the CRM 126-A and the other IDMS reverse-spikes were determined from multiple measurements on calibrated balances that were checked with certified weight sets prior to weighing and were corrected for air buoyancy based on laboratory-specific conditions.
Dilution of the IRMM 086 reference material was necessary to prepare the reverse-spike solution for IDMS analysis. The CRM unit was comprised of a screw-cap glass ampoule containing approximately 22 µmol of 0.97 n(239Pu)/n(Pu) plutonium in 5 mL of a 5 mol L−1 HNO3 solution. The IRMM 086 solution was transferred from the original ampoule to a 1 L FEP bottle and approximately 1 L of a mixed 4 mol L−1 HNO3 and 0.05 mol L−1 HF solution was then added to bottle. To achieve an appropriate concentration for characterization of the stock solution, a 6 g aliquot of the diluted IRMM 086 solution was transferred to a 250 mL FEP bottle and 250 mL of a 4 mol L−1 HNO3 and 0.05 mol L−1 HF solution was added to create IRMM 086 Dilution 1.
A third IDMS reverse-spike solution was prepared by adding approximately 30 mL of a 4 mol L−1 HNO3 and 0.05 mol L−1 HF solution to a new unit of CRM 130 (which is composed of 1 mg of 0.999 n(242Pu)/n(Pu) enriched plutonium as a dry nitrate in a 30 mL FEP bottle). A 2.9 g aliquot of the CRM 130 solution was transferred to a 1 L bottle and diluted with approximately 1 L of a 4 mol L−1 HNO3 and 0.05 mol L−1 HF solution to create CRM 130 Dilution 1 at a concentration appropriate for spiking the 244Pu stock solution.
Aliquots of the IDMS reverse-spikes were added to 18 randomly selected units of the 244Pu Spike (6 units for each reverse-spike). Additionally, an aliquot of the CRM 126-A Dilution 1 was added to the “A” analysis sample and IRMM 086 Dilution 1 was added to the “B” analysis sample. The masses of the reverse-spike aliquots were carefully measured by difference on the 5-place XPE105 balance using disposable HDPE pycnometers (CANUS Plastics, Ottawa, Canada) with capillary tips; allowing for highly reliable solution mass measurements with minimal potential for bias due to evaporation. After spiking, the A and B solutions were subdivided into three equal volumes. The subsamples of A and B and the reference material units prepared with CRM 126-A Dilution 1 and IRMM 086 Dilution 1 reverse-spikes were then distributed among the three mass spectrometry laboratories for plutonium isotopic analysis. The reference material units prepared with CRM 130 Dilution 1 were analyzed at LLNL.
Pu isotope amount ratio measurements
Measurements of the plutonium isotopic composition for the 244Pu Spike and the IDMS samples were performed by mass spectrometry facilities at LLNL, LANL, and CEA. In addition to the mixed IDMS samples, each laboratory was provided with an unaltered unit of the 244Pu Spike for the plutonium isotopic composition measurements (Unit 52 for LLNL, 90 for LANL, and 171 for CEA). All three laboratories made measurements on magnetic-sector multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS) instruments. A single facility, LANL, made additional isotopic composition analyses using a total evaporation method on a multi-collector thermal ionization mass spectrometer (MC-TIMS). Separations were performed on the isotopic composition samples to remove ingrown 241Am prior to analysis. Replicate analyses were performed for both IDMS and isotopic composition and mass spectrometers were calibrated using certified reference materials. Table 2 is a summary of the instruments, methods, and calibrations used for plutonium mass spectrometric measurements performed at the three laboratories.
Results
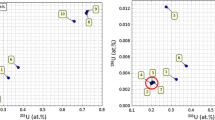
The results for the 244Pu Spike isotopic composition measurements are summarized in Table 3 and individual analysis results are shown in Fig. 3. All three analysis laboratories made multiple mass spectrometry measurements of the n(239Pu)/n(244Pu), n(240Pu)/n(244Pu), n(241Pu)/n(244Pu), and n(242Pu)/n(244Pu) ratios. The measurement data are variable but consistently show that the 244Pu material is highly enriched, having a 244Pu atom fraction of greater than 0.9998 n(244Pu)/n(Pu). Analysis of Variance (ANOVA) performed on the data sets indicate that there is a laboratory-to-laboratory bias at a 95% level of confidence for MC-ICP-MS measurements of each isotopic ratio (F statistics ranging from 5.3 to 25 for an F critical of 4.7).
Plutonium isotope amount ratio data for individual measurements. Error bars are expanded uncertainties (k = 2) as reported by the analysis laboratories. For some measurement result the error bars are smaller than the symbols. The solid lines represent mean attribute values calculated for each isotope amount ratio and the dashed lines delineate an expanded uncertainty envelope (k = 2) for the mean value. All values are decay corrected to a reference date of 8 March 2017
For information purposes, LLNL performed duplicate n(238Pu)/n(244Pu) isotopic ratio measurements by alpha spectrometry. These measurements yielded an average value of 6.45 × 10−7 with a 0.11 × 10−7 combined standard uncertainty (k = 1). LANL measured the n(238Pu)/n(244Pu) isotopic ratio by ion counting on a TIMS instrument using a method that effectively minimizes the potential for 238U isobaric interferences [19]. These measurements indicated an average n(238Pu)/n(244Pu) value of 8.09 × 10−7 with a 0.32 × 10−7 combined standard uncertainty (k = 1). The higher measured ratio for the TIMS data is consistent with biases observed for this analysis method when measuring < 1 fg quantities of 238Pu (TIMS sample loads for this project contained approximately 0.3 fg of 238Pu).
The measurement results for molality of 244Pu in the stock solution are provided in Table 4 and shown in Fig. 4. IDMS measurements using the CRM 126-A Dilution 1 and the CRM 130 Dilution 1 reverse-spikes have mean values that are essentially indistinguishable but there is a consistent bias (− 0.34% relative) between the IRMM 086 Dilution 1 reverse-spike results and the other two data sets. ANOVA performed on measurements results for molality 244Pu made with the CRM 126-A Dilution 1 indicate a statistically significant laboratory-to-laboratory difference (F statistics of 7.9 for an F critical of 5.14) whereas data for IRMM 086 show a similar pattern of variability but do not indicate a significant difference between the data sets (F statistic of 4.75 for an F critical of 5.14). This pattern of between-laboratory variability is also reflected in the distribution of the result from both the A and B test samples (i.e., individual IDMS solutions split for analysis at all three laboratories). The verification IDMS measurements using CRM 130 Dilution 1 were made under repeatability conditions, as defined in [20], and have relative standard deviation of 0.065%. These data appear to decrease with fill number, but the magnitude of this trend is smaller than the uncertainties for the individual measurements and the trend is not reflected in molality measurements results for the other IDMS reverse-spikes.
Characterization and verification measurement data for the molality of 244Pu in the 244Pu Spike stock solution. Error bars are expanded uncertainties calculated for individual measurements (k = 2). The solid line represents the mean attribute value calculated for the molality of 244Pu and the dashed lines delineate an expanded uncertainty envelope (k = 2) for the mean value. As specified in the legend, the shape of the data markers indicate which laboratory performed the mass spectrometry measurements. Note that the unit numbers correspond to the unit filling order
Discussion
A high-quality reference material is required to be stable and homogeneous and to have specified attribute values that have been verified, are metrologically traceable, and have appropriate measurement uncertainties [21]. The 244Pu Spike project was planned and executed specifically to meet these requirements. A detailed discussion of the measurement results, attribute value determinations, and measurement uncertainties for the 244Pu Spike is provided below.
Stability
The stability of the 244Pu spike reference material is considered to be indefinite in that temporal changes are not anticipated to increase uncertainties of the characterized attribute values or diminish the usefulness of the reference material units for a period that significantly exceeds the planned lifetime for the production run (20 a). The 244Pu nuclide has a half-life of 80 Ma, so changes in the amount of 244Pu in a unit due to radioactive decay will be indiscernible over the course of decades. The relative proportions of the other plutonium isotopes to 244Pu will, however, change significantly over useful life of the spike but these changes are predictable, easily corrected for, and not detrimental to the utility of the 244Pu Spike.
The 244Pu Spike units were prepared by dispensing 5 mL of an acid solution into 30 mL FEP bottles. The molality of plutonium in the aqueous solution in these units could change significantly due to evaporation over a relative short period of time (weeks to months). Accordingly, every reference material unit was dried-down after it was carefully weighed, resulting in the plutonium being entrained within a viscous H3PO4 film on the bottom of each container. The advantage of providing the plutonium amount as an absolute quantity is that any changes to the form of the material within the primary container (such as hydration or desiccation of the H3PO4 film) will not alter the characterized attribute value. In this form, it is anticipated that the 244Pu Spike units will remain fit-for-purpose provided that the units are properly stored and handled, and appropriate decay corrections are applied to the characterized plutonium isotope amount ratios.
Homogeneity
Heterogeneous reference material units would significantly diminish the utility of the 244Pu Spike reference material. The stock solution dispensed to the 244Pu Spike units was thoroughly mixed to assure homogeneity and was dispensed in a single 3 h period to minimize the opportunity for changes during handling. As described in the Reference Material Production section, considerable effort was taken to prevent differential contamination with extraneous plutonium prior to and during unit production. The plutonium blank measurements performed in association with this project indicate that these efforts were successful.
To verify that the reference material is homogeneous, measurements of the molality of 244Pu were made on 20 separate samples, including 18 reference material units and 2 special samples. The resulting data is adequate to assess measurement variability for the production run. The data do not, however, have the resolution necessary to distinguish between-unit heterogeneity due to the inherent difficulty of analyzing the relatively small amounts of plutonium material in each unit (≈ 120 ng). To appropriately assess unit-to-unit homogeneity, multiple high-precision analyses for individual sample unit are necessary to constrain the magnitude of measurement repeatability. Small samples are not conducive to multiple high-precision measurements due to limits on the types of measurement techniques that can be applied, the duration of analyses, and signal intensities during measurements. Two larger samples (A and B) were prepared, but these were distributed specifically to assess laboratory-to-laboratory variability in the measurement results for molality of 244Pu. The six units of the 244Pu Spike that were analyzed under repeatability conditions (CRM 131 Dilution 1 reverse-spike samples) provide data to assess within laboratory measurement variability in addition to the replicate samples analyzed using the CRM 126-A and IRMM 086 reverse-spikes. Within-laboratory variability for measurements of the molality of 244Pu is consistently smaller (0.03 to 0.07% RSD) than the between-laboratory variability for the distributed A and B samples (0.10 and 0.08% RSD respectively). This observation is also consistent with the ANOVA performed on the CRM 126-A reverse-spike data which shows that there is a statistically significant laboratory-to-laboratory bias between IDMS measurements. Accordingly, the molality of 244Pu in the solution dispensed to the reference material units appears to be homogenous within the resolution of the measurement data and heterogeneity, if any, is smaller than the observed laboratory-to-laboratory variability which is specifically incorporated into the uncertainty budget for the 244Pu Spike.
Isotope amount ratio data are usually a sensitive indicator of contamination or heterogeneity, however the purity of 244Pu material and the relatively small quantities of plutonium available for analysis made high-precision isotopic ratio measurements difficult. For example, the within-laboratory variability for the n(239Pu)/n(244Pu) ratio as measured on a single sample unit, ranges from 0.5 to 1.5% RSD and the method-to-method bias for analyses of a single sample (LANL data) is almost 5%. Considering the limited resolution of measured isotopic ratios for low abundance plutonium isotopes, the detection of heterogeneity in the 244Pu Spike would only be possible in the unlikely event of gross contamination.
As previously noted, the molality of plutonium for measurements made with the CRM 130 spike appears to decrease systematically with fill order. Yet the magnitude of this decrease is smaller than the measurement uncertainties and no correlation of molality of plutonium with the fill order is observed for either the CRM 126-A or the IRMM 086 reverse-spike samples analyzed at any of the three mass spectrometry laboratories (Fig. 4). Accordingly, the trend observed in the CRM 130 Dilution 1 samples is likely to be happenstance or is related to preparation of the IDMS samples (sample spiking was performed in ascending order). Considering the ambiguity of the evidence for a fill-order dependent trend and the relatively small magnitude of the variability, no additional uncertainty component was applied to the data.
Traceability
In order to establish the traceability of the characterized attributes for the 244Pu Spike to the SI it is necessary to demonstrate that “the result can be related to a reference through a documented unbroken chain of calibrations, each contributing to the measurement uncertainty” [21]. The attributes characterized for this material are the 244Pu amount and the n(239Pu)/n(244Pu), n(240Pu)/n(244Pu), n(241Pu)/n(244Pu), and n(242Pu)/n(244Pu) isotope amount ratios. Figure 5 is a schematic showing how the characterized values for the 244Pu amount and plutonium isotope amount ratios can be tied directly to the mole through calibrations using reference materials that were either certified or originally characterized by the National Institute of Standards and Technology, the Institute of Reference Materials and Measurements (IRMM) or by New Brunswick Laboratory (the DOE organization maintaining nuclear material standards for the USA).
Measurements of plutonium isotope amount ratios were made by MC-ICP-MS and, for one laboratory, by TIMS. Reference materials certified for isotope-amount ratios were used to correct measured isotope amount ratios for mass bias and for detector intercalibrations (i.e. secondary electron multipliers, Daly detector, Faraday cups). Mass spectrometer calibrations were verified by quality control measurement of certified reference materials, including CRM 138, CRM 137, CRM 126-A, and IRMM 086.
Isotope dilution mass spectrometry is a primary method for determination of molality assuming that input variables are appropriately constrained [22, 23]. The primary variables for the IDMS measurements of the 244Pu stock solution are the measured plutonium isotope amount ratios, the plutonium molality of the reverse-spike, and masses of the reverse-spike and sample aliquots. All weighing operations were performed on calibrated balances that were checked before and after project activities using certified weight sets; a certified plutonium assay and isotopic standard, CRM 126-A, was used as a reverse-spike; and the measured plutonium isotope amount ratio measurements are traceable through instrument calibrations using certified reference materials. Therefore, the resulting molality measurement for the 244Pu Spike stock solution is traceable. Using the mean mass value for aliquots of the stock solution dispensed into each unit container allows for a traceable amount of substance to be specified for the reference material.
Verification
The measured molality of 244Pu for the 244Pu Spike stock solution was verified by making independent measurements under reproducibility conditions, as defined in [20]. The mean 244Pu stock solution measurement results are essentially identical for samples spiked with CRM 126-A or CRM 130. This verification is robust considering that the plutonium IDMS spikes were prepared from substantially different starting materials (0.93 atom fraction 239Pu metal vs 0.999 atom fraction 242Pu nitrate) that were used in separate measurement campaigns (preparation of CRM 130 IDMS spike, preparation of the IDMS samples, and the mass spectrometry analyses were performed 6 months after the CRM 126-A IDMS analyses).
Measurements of the 244Pu Spike using the IRMM 086 reverse-spike do not verify the CRM 130 and CRM 126-A IDMS data but have an average relative bias of − 0.34% from the. The most likely explanations for this bias are that the IRMM 086 unit procured for this project was slightly more concentrated than the certificate value or there was a misstep during reverse-spike preparation. There is no independent evidence of an issue with either the IRMM 086 unit or the reverse-spike preparation, but it is notable that units of IRMM 086 were prepared in screw-top ampoules approximately 10 a prior to use in this project. Evaporative loss through the seals of a screw-top closure is plausible. Evaporation of only 17 µL of solution from the CRM unit over the course of a decade would result in a 0.34% increase in the plutonium concentration of the solution which could fully account for the observed bias in the IDMS data.
The reproducibility of the plutonium isotopic composition of the 244Pu Spike was assured by using measurement data from three independent laboratories (4 different instruments) to define the characterized attribute values. Laboratory-to-laboratory variability is observed for the isotope ratio measurements, but the measurements are reasonably consistent and some degree of measurement variability was anticipated considering the extreme isotopic ratios (< 1:10 000 for all plutonium isotopes relative to 244Pu). The mean values calculated for data from the 3 laboratories incorporate uncertainty components for both within-laboratory/method and between-laboratory/method variability along with various systematic uncertainty components (see proceeding section).
Attribute values and measurement uncertainty
Attribute values for the 244Pu Spike reference material were determined for the amount of 244Pu in a unit and for plutonium isotope amount ratios (Table 5). The molality of 244Pu in the stock solution dispensed to the unit containers was characterized for this study with the intent of providing unique amount values for each unit. After the unit weighing was completed, it was observed that the maximum difference between masses for the aliquots of the stock solution is 0.0037 g which is 0.066% relative to the average mass of the dispensed solution. This relative difference is similar in magnitude to the relative standard deviation of the stock solution molality analyses (i.e. 0.069%) and represents only a small proportion of the total measurement uncertainty (see Table 6). Accordingly, the difference in amount of plutonium per unit is considerably smaller than the uncertainty for each unit resulting in a single average value for amount of 244Pu being applicable to every unit of the reference material production run. Unit-specific container masses will be provided to users to further facilitate accurate calculations of molality of 244Pu during preparation of the 244Pu Spike for use.
Uncertainty models for the characterized attributes were developed in accordance with the requirements of the ISO Guide for the Expression of Uncertainty in Measurement [24] and NIST Technical Note 1297 [25]. The GUM Workbench software [26] was used to calculate the final attributes values and prepare uncertainty budgets. The uncertainty for the 244Pu amount per unit (Table 6) is dominated by the Type-B evaluated uncertainty estimated for the IDMS isotopic ratio measurements (RMassSpec: including blank corrections, background corrections, measurement repeatability, and cup efficiencies) and the Type-A evaluated component representing between-laboratory variability (NVarB) of the IDMS data, as derived from ANOVA. The Type-A evaluated within-laboratory variability (NVarW) for the molality of 244Pu, the Type-B evaluated uncertainty components for the average mass of the stock solution dispensed to the units (MUnitAve), the mass of IDMS aliquots (MAliquot), the certificate values for the mass spectrometry calibration CRMs (RCRM), and the concentration of the CRM 126-A Dilution 1 reverse-spike (CC126-A) also contributed to the overall uncertainty. Other potential components did not contribute significantly (< 0.01% relative standard uncertainty) to the overall uncertainty for the characterized amount of 244Pu per unit. These included uncertainties for the plutonium isotopic compositions of the 244Pu Spike and the CRM 126-A reverse-spike.
The characterized plutonium isotope amount ratio values for the 244Pu Spike were calculated as the mean value of the isotope amount ratios measured by the three laboratories. The combined uncertainties for the characterized plutonium isotope amount ratios (Table 7) are dominated by Type-A evaluated uncertainty components representing the between-laboratory variability (RVarB), as derived from ANOVA, for the respective measurement data sets. The Type-A evaluated component representing the with-laboratory variability (RVarW) for the isotope amount ratios, the Type-B evaluated uncertainty estimate for the mass spectrometry measurements process (RCorr: which includes blank corrections, background corrections, measurement repeatability, and detector intercalibrations), and the mass spectrometry calibration CRMs (RCRM) also contribute to the overall uncertainty. Uncertainty components for decay correction of the plutonium isotopic measurements to a common reference date (δdecay) represent a minimal contribution to the uncertainty for the characterized n(241Pu)/n(244Pu) ratio and are insignificant for the other isotopic ratios.
Conclusion
Measurement results for the molality of 244Pu in the stock solution used to produce the 244Pu Spike reference material units are consistent, traceable, reproducible, and indicate that the solution was homogeneous. The variability in the mass of solution dispensed to each unit container was relatively small for the entire production run allowing a single amount value to be assigned to the 244Pu Spike units. The plutonium isotope amount ratio measurement results are variable due to the very low abundances of the other plutonium isotopes relative to 244Pu and the limited size of the analyzed samples. Nevertheless, the uncertainties resulting from this variability do not impair the utility of the spike. The highly-enriched 244Pu Spike will enable precise measurements of the amount and isotopic composition of plutonium to made from a single mass spectrometric analysis. Accordingly, the 244Pu Spike is a suitable IDMS reference material for use in measurements frequently performed for nuclear forensics and nuclear safeguards.
Notes
Certain commercial equipment, instruments, software, or materials are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
References
Tuli JK (2011) Nuclear wallet cards, national nuclear data center, https://www.nndc.bnl.gov. Accessed 12 Dec 2018
Hoffman DC, Lawrence FO, Mewherter JL, Rourke M (1971) Detection of Plutonium-244 in Nature. Nature 234:132–134
Steier P, Hornecek E, Priller A, Quinto F, Srncik M, Wallner A, Wallner G, Winkler S (2013) AMS of minor plutonium isotopes. Nucl Instum Methods Phys Res 294:160–164
Armstrong CR, Brant HA, Nuessle PR, Hall G, Cadieux JR (2016) Anthropogenic plutonium-244 in the environment: insight into plutonium’s longest lived isotope. Sci Rep. https://doi.org/10.1038/srep21512
NBL (1987) CRM 131 Plutonium-244 in Nitrate Form (Plutonium Spike Assay and Isotopic Standard). NBL Program Office, Lemont, IL
IRMM (2017) Certified Reference material IRMM—042a. Joint research center—geel, Geel, Belgium. Certificate available at https://crm.jrc.ec.europa.eu. Accessed 31 Jan 2017
IAEA (2011) Quantification procedure for the network of analytical laboratories for environmental sampling, International Atomic Energy Agency, SG-SGAS-9006, Version1
Deron S, Vesnovskii S (1999) Development of technology for high-purity production by method of electromagnetic separation. Nucl Instum Methods Phys Res. 438:20–22
Penkin MV, Humphrey MA, Kryzhanovsky AA, Vyachin VN, Iyengar A (2016) Separation of high-purity 244Pu for safeguards application. J Radioanal Nuc Chem 307:2091–2094
Harley JH (1980) Plutonium in the environment—a review. J Radiat Res 21:83–104
NBL (2004) CRM 126-A plutonium metal assay and isotopic standard. NBL Program Office, Lemont, IL
IRMM (2007) Isotopic reference material IRMM—086. Joint research center—geel, Geel, Belgium. Certificate available at https://crm.jrc.ec.europa.eu. Accessed 31 Jan 2017
NBL (1987) CRM 130 Plutonium-242 spike assay and isotopic standard in nitrate form. NBL Program Office, Lemont, IL
NBL (2008) CRM 128 plutonium-239/plutonium-242 1:1 atom ratio plutonium isotopic standard in nitrate form. NBL Program Office, Lemont, IL
NBL (1987) CRM 137 Plutonium isotopic standard. NBL Program Office, Lemont, IL
NBL (1987) CRM 138 plutonium isotopic standard. NBL Program Office, Lemont, IL
IRMM (2005) Isotopic reference material IRMM—186. Joint research center—geel, Geel, Belgium. Certificate available at https://crm.jrc.ec.europa.eu. Accessed 10 July 2018
NBL (2003) CRM U005-A uranium isotopic standard. NBL Program Office, Lemont, IL
Maassen J, Inglis JD, Wende A, Kayzar-Boggs TM, Steiner RE, Kara A (2019) Analysis of sub-picogram quantities of 238Pu by thermal ionization mass spectrometry. J Radioanal Nuc Chem 321:1073–1080
JCGM (2012) International vocabulary of metrology—basic and general concepts and terms (VIM). JCGM-Joint Committee for Guides in Metrology. JCGM 200, 2012
ISO (2017) Reference materials—guidance for characterization and assessment of homogeneity and stability. The International Organization for Standardization. ISO Guide 35:2017(E)
Milton MJT, Quinn TJ (2001) Primary Methods for the measurement of amount of substance. Metrologia 38:289–296
De Bièvre P, Peiser HS (1997) Basic equations and uncertainties for isotope-dilution mass spectrometry for traceability to SI of values obtained by this primary method. Fresenius J Anal Chem 359:523–525
JCGM (2008) Evaluation of measurement data—Guide to the expression of uncertainty in measurement. JCGM 100, (E/F)
Taylor BN, Kuyatt CE (1994) Guidelines for evaluating and expressing the uncertainty of NIST Measurement results: NIST technical note 1297. National Institute of Standards and Technology, Gaithersburg
GmbH Metrodata (2009) GUM Workbench. Weil am Rhein, Germany
Acknowledgements
Reference material preparation and production at LLNL and characterization measurements and LLNL and LANL were funded by the United State National Nuclear Security Administration. Project activities at NIST were supported by the United States Department of Homeland Security. The analyses performed at CEA were supported internally.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Richard M. Essex, Ross W. Williams, Kerri C. Treinen, Amélie Hubert, Marc A. Humphrey, Jeremy D. Inglis, William S. Kinman, Joel Maassen, Maxim V. Peńkin, and Robert E. Steiner declare that they have no conflict of interest.
Human/animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Essex, R.M., Williams, R.W., Treinen, K.C. et al. A highly-enriched 244Pu reference material for nuclear safeguards and nuclear forensics measurements. J Radioanal Nucl Chem 324, 257–270 (2020). https://doi.org/10.1007/s10967-020-07075-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07075-y