Abstract
Radio-chromatography was conducted by using the astatine radionuclides 209,210,211At produced in the 7Li induced reaction of 209Bi. The speciation of dissolved astatine chemical species of astatide (At−), astatate (AtO3−) and perastatate (AtO4−) was carried out by thin layer chromatography on silica gel with an ethanol/water solution. Amounts of the astatine species varied with concentrations of oxidizing and reducing reagents, potassium periodate, sodium sulfate and hydrazine hydrate. The oxidation–reduction reactions between At−(−I) and AtO3− (V) were found to form volatile At0 (0) and release it from a silica gel thin layer in the development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
211At, an astatine radionuclide, with a half-life of 7.2 h is one of the prospective candidates for utilization in targeted alpha therapy of cancers. Astatine shows some different chemical behaviors in comparison with its homologue iodine. The understanding of basic chemical properties of astatine has been required to develop targeted alpha therapy reagents for cancers [1].
The speciation of astatine has been studied [2,3,4,5,6,7,8,9,10,11,12,13,14] but low amounts of astatine present could cause the difficulty in experimental identification [1]. In addition, identification of chemical species is generally affected by the preparation of samples and analytical methods. Some astatine chemical species in solutions were suggested; the identification of At−, At+ and AtO3− was proposed by Appelman [3] and supported by Rössler et al. [4]. The different identification of At−, At+ and AtO+ has recently been proposed by Champion et al. [11]. In contrast, At−, AtO3− and AtO4− were separated and identified with paper electrophoresis and paper chromatography by Dreyer et al. [5]. Two of the three astatine species, At− and AtO3−, were identified with high-performance liquid chromatography (HPLC) by Balkin et al. [13].
In our previous study of thin layer chromatography (TLC) [14], the identification of the three astatine species has recently confirmed. The astatine speciation was carried out by comparing with retardation factors (Rf) of iodine anions. Furthermore, the behavior of the relative amounts of the astatine species with the oxidizing and reducing reagents, potassium periodate (KIO4), sodium sulfate (Na2SO3) and hydrazine hydrate (NH2NH2·H2O), revealed that the astatine species were certainly identified as At−, AtO3− and AtO4−. The oxidation numbers of astatine are At(−I) for At−, At(V) for AtO3− and At(VII) for AtO4−. In the case of the TLC analysis for the solutions containing the reducing reagent of hydrazine hydrate, a marked loss of astatine activities in the development was observed. This observation suggests that the oxidation–reduction reactions between At−(−I) and AtO3−(V) in dynamic equilibria enhance the probability to form At0(0) characterized by high volatility [3] and release it from a silica gel thin layer in the development. The understanding the production of the volatile astatine species is required to conduct TLC experiments from a perspective of radiation safety.
The chemical nature of the volatile astatine species from solutions still remains uncertain. Distillation of astatine solution was found to take place under reduction conditions (0.05 M FeSO4 in 0.5 M H2SO4) by Johnson et al. [2]. Such distillation technique under reductive conditions (0.75 M FeSO4 in 1.5 M H2SO4) has recently used to purify 211At from basic solutions; it is presumed that hydroastatic acid is distilled [13].
The aim of this work is to elucidate the relation between the production of the volatile species of At0 and the oxidation–reduction behaviors among three dissolved astatine species of At−, AtO3− and AtO4− in terms of not only radiation safety but also the fundamental interest in chemical properties of astatine. The TLC analysis for dissolved and volatile astatine species has been conducted by varying concentrations of KIO4, Na2SO3 and hydrazine.
Experimental
Production of radionuclides
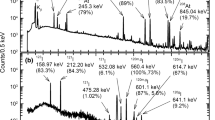
The astatine radionuclides were produced via the nuclear reaction of natPb(7Li,xn) 209,210,211At [15]. A stack of thin metal lead targets of approximately 1 mg/cm2 in thickness was irradiated with 60 MeV 7Li3+ ion beams supplied from the Japan Atomic Energy Agency (JAEA) tandem accelerator. Beam current was controlled to be approximately 180 nA during the irradiation of 3 h. After irradiation, radioactivity of astatine radionuclides 209,210At produced in the target was measured by a high-purity germanium detector. Such target preparation, irradiation, γ-ray measurements as well as the preparation of astatine aqueous solutions described below were carried out in the similar way as described in our previous study [14].
Preparation of astatine aqueous solutions
The produced astatine radionuclides were separated from the irradiated targets by dry distillation. The dry distillation was conducted in the 180 × 18 mm (o.d.) glass test tube replaced air with nitrogen gas and sealed with polyethylene films. A third portion from the bottom of the test tube was inserted into a furnace at 650 °C for 20 min. After dry distillation, no-carrier-added astatine aqueous solutions were prepared as mother stock solutions by eluting the separated astatine radionuclides with 1.8 mL of distilled water. The activity of 211At in the mother stock solutions was determined by α-ray spectrometry as described by Nishinaka et al. [15]. The sources for α-ray spectrometry were prepared by depositing a 5 μL portion of the astatine aqueous solutions on a silver sheet and evaporating them. Alpha particles from the sources were detected with a silicon surface barrier detector (ORTEC).
Preparation of astatine solutions containing an oxidizing or a reducing reagent in a concentration range
In order to investigate oxidation–reduction degree of astatine species, astatine in the mother stock solution was reacted with an oxidizing and two single reducing reagents in a concentration range as follows: (1) For oxidation by KIO4, reaction mixtures were prepared by adding 50 μL KIO4 solutions (3 × 10−5, 3 × 10−4, 3 × 10−3 and 3 × 10−2 mol L−1) or 50 μL H2O into 200 μL of the astatine aqueous solution as mother stock solution, and heating at 90 °C for 20 min. (2) For Na2SO3, reaction mixtures were prepared by adding 25 μL Na2SO3 solutions (5 × 10−4, 5 × 10−3, 5 × 10−2 and 5 × 10−1 mol L−1) or 25 μL H2O into 100 μL of the astatine aqueous solution as mother stock solution, and were kept at ambient temperature for 20 min. (3) For hydrazine, reaction mixtures were prepared by adding 10, 20, 30 and 40 μL NH2NH2·H2O solutions into 100 μL of the astatine aqueous solution, and were kept at ambient temperature for 20 min. The radioactivity concentrations of the reaction mixtures are different in the case of (3).
TLC for astatine
Thin layer chromatographic isolation was achieved in the same way described in our previous study [14]. A 5 μL portion of the reaction mixtures was spotted at 2 cm from the bottom of the silica gel TLC plate cut in strips 12 × 2 cm. A solvent mixture of ethanol/water (1:1, v/v) ascended the TLC plate in the distance of 8 cm from the spotted point. Distributions of activity on the TLC plate were measured with imaging plates and visualized by Bioimaging Analyzer System (BAS) (BAS-5000, FUJIFILM Life Science). All TLC plates each for KIO4, Na2SO3 and hydrazine, and a plate for the corresponding astatine aqueous solution were exposed to an imaging plate. This enabled us to quantitaively compare the amounts of the astatin chemical spiecies reacted with each the oxidizing and reducing reagents in the concentration range.
Results and discussion
TLC successfully separated spots of astatine anions as shown in Figs. 1, 2, 3. Figures 1, 2, 3 each aligned the parts of the same BAS image in the area contacted with the TLC plates. The spots at the origin of the TLC plates correspond to AtO4−(VII), and the lower and the upper bands correspond to AtO3−(V) and At−(−I), respectively, as identified in Ref. [14].
The amounts of astatine radionuclides in the astatine aqueous solution at the time of contacting the TLC plates to an imaging plate were 26 ± 1 Bq 209At, 44 ± 1 Bq 210At and 33 ± 1 Bq 211At for Fig. 1a, 19 ± 1 Bq 209At, 26 ± 1 Bq 210At and 34 ± 1 Bq 211At for Fig. 2a, and 50 ± 2 Bq 209At, 108 ± 3 Bq 210At and 11 ± 1 Bq 211At for Fig. 3a, respectively. These values were corrected for decay time from measuring activities to contacting the TLC plates to an imaging plate. It should be noted that TLC analysis was accomplished using the low amounts of no-carrier-added astatine present.
Images of Fig. 1 show that the increase in KIO4 concentration heightens the intensity of the spots of AtO4−(VII) at the origin of TLC, and slightly lowers that of the bands of At−(−I) and AtO3−(V), indicating the oxidation of astatine. In contrast, the reduction of astatine with increasing in concentrations of Na2SO3 and hydrazine is apparently observable as decreasing and disappearing the intensity of the spots of AtO4−, in Figs. 2 and 3, respectively. Additionally, loss of astatine activity is visible for hydrazine (Fig. 3).
Component analysis
To quantitate degrees of oxidation–reduction, a component analysis was carried out by using the chromatograms obtained from the BAS images in Figs. 1, 2, 3. Typical chromatograms are displayed in Fig. 4a–c. Figure 4a–c correspond to the BAS images for KIO4 in Fig. 1a, e, those for Na2SO3 in Fig. 2a, d, and those for hydrazine in Fig. 3ad, respectively. Distributions of astatine activities on a plate are shown by intensity of a BAS image as a function of Rf. Solid and dashed lines correspond to the astatine aqueous solution with and without the oxidizing or the reducing reagent, respectively. The background level shown by dash-dotted line was taken from the average intensity of several areas of the BAS image. The intensities were normalized by the factor as the total intensity in the range of Rf = –0.25–1.25 for the corresponding astatine aqueous solution becames 100%.
Relative amounts of astatine species were obtained by integrating the normarized intensity in the ranges of Rf = –0.08–0.08 for AtO4−, 0.64–0.82 for AtO3− and 0.82–1.00 for At−. These Rf ranges are shown by gray vertical line in Fig. 4. The results of the component analysis for KIO4, Na2SO3 and hydrazine were listed in Tables 1, 2, 3, respectively. The relative amounts for hydrazine were also corrected for the radioactivity concentrations in the solutions. Errors of relative amounts not shown in the Tables 1, 2, 3 were estimated to be the sensitivity for non-uniformity of BAS images (∼ 7%).
Finally, it should be noted that the aqueous solutions show rather different composition profiles of the relative amounts of At−, AtO3− and AtO4− among trials. The composition range of 16–26% in this work is slightly small compared with that of 18–48% for an aqueous solution in Ref. [14]. The reason of the composition variation for aqueous solutions is not clear but this means that astatine is easily oxidized and reduced in water due to the more electropositive character of astatine than iodine [14]. This implys that such composition variation of At−, AtO3− and AtO4− in no-carrier added astatine solution prepared by dry-distillation would conduct problematic results [16] in the repeatability of yields in the chemical and radiolabeling reactions.
Missing amounts
The loss of astatine activity was clearly observed in the component analysis. In order to investigate the loss of astatine activity from TLC plate, missing amount was defined and obtained as difference of the total intensity for the chromatograms between the astatine solutions with and without the oxidizing or the reducing reagent. The results are listed in the column of the missing amount in Tables 1, 2, 3.
The missing amounts assume that At0 characterized by high volatility [3] is not released from TLC plates for aqueous solutions without the oxidizing or the reducing reagent. This assumption makes a simple consideration of the loss of astatine activity as At0(0) forms via the oxidation–reduction reaction between At− (−I) and AtO3−(V) in dynamic equilibria and volatilizes from TLC plates as pointed out in our previous study [14]. From the results of the missing amounts, not only the volatilization of At0 but also validity and ambiguity of such asummption of no release for astatine solution without the oxidizing or the reducing reagent will be discussed as follows.
In Tables 1 and 2 for KIO4 and Na2SO3, the missing amounts range from − 18 to 20% and seem to have no correlation with the reagent concentrations. Thus, the range of these irregulary fluctuated missing amounts (∼ 20%) can be regarded as a systematic error in this work. The systematic error originates from experimental and analytical conditions; non-uniformity of BAS images (∼ 7%) will be a contributing factor for the systematic error. In addition, one of the other possible factors is volatilization of astatine At0 from TLC plates not only for KIO4 and Na2SO3 solutions but also for aqueous solutions. At0 strictly have a chance to form and volatilize from TLC plates even for aqueous solutions. Because the presence of tailing in the region of Rf = 0.08–0.64 in Fig. 4 clearly shows that oxidation–reduction reactions between astatine species in dynamic equilibria will occur in the development. Thus, the contribution of At0 can not be negligible but can be estimated within the systematic error range of approximately 20%.
In Table 3, in contrast to that, the missing amount for hydrazine significantly increases with the volume ratio over the systematic error range (∼ 20%) estimated for KIO4 and Na2SO3. This indicates that these large missing amounts come from the formation of At0 due to the presence of the reducing reagent of hydrazine, therefore the missing amounts are assigned to the relative amounts of At0 in Table 3. This aspect will be discussed in more detail later.
Dependence of astatine anion yields on concentration of reagents
Figures 5, 6, 7 display that the relation between relative amounts of astatine species with concentrations of KIO4, Na2SO3 and hydrazine listed in Tables 1, 2, 3, respectively.
As shown in Fig. 5, an increase in KIO4 concentration enhances relative amounts of AtO4− up to more than 30%. In contrast, relative amouts of At− and AtO3− both stay around 20% at low concentrations and largely drop below 10% at the highest concentration of 6 × 10−3 mol L−1. The order of relative amounts is consistent with that of the oxidation number, AtO4−(VII) > AtO3−(V) > At−(−I). This dependence of the astatine anion amounts on concentration of KIO4 obviously indicates the oxidation of astatine and the reliability of the speciation of astatine [13].
Figure 6 shows that the reducing reagent of Na2SO3 decreases the relative amounts of AtO4− down to less than 20% while yields of At− and AtO3− stay between 20 and 30%. The order of the amounts is roughly good agreement with the inverse order of the oxidation number, At−(−I) ≥ AtO3−(V) >AtO4−(VII). However, no increasing behavior of missing amounts with concentration was observed. This concentration dependence shows that Na2SO3 seems to act as a weak reducing reagent.
In the case of hydrazine, the concentration is presented in volume ratio (Fig. 7). Data for the astatine aquesous solution are plotted at the origin for horizontal axes. The amounts listed in the coloum of At0 (missing amount) in Table 3 are shown by solid circle. The presence of hydrazine rapidly suppresses the relative amount of AtO4−. The AtO4− species is vanished and completely reduced to the lower oxidation states of AtO3− and At− over 23.1% in volume ratio. In contrast, the relative amounts of AtO3− and At− slightly increase with the volume ratio of hydrazine at lower concentrations, and then appearently decrease over 16.7% in volume ratio, and finally become less than 20% at 28.6% in volume ratio. Such decreases in the relative amounts of not only AtO4− but also AtO3− and At− with hydrazine concentration connect to a large increase of At0.
The observed concentration dependence of the relative amounts of not only AtO4−, AtO3− and At− but also At0 visibly supports the interpretation in the previous study [14]; The reduction of AtO4−(VII) with hydrazine concentration enhances the relative amounts of AtO3−(V) and At−(−I). This facilitates the formation of At0(0) through the oxidation–reduction reaction between AtO3−(V) and At−(−I) in dynamical equilibria due to both effects of the oxidation on silica gels and the reduction with hydrazine, leading to the volatilization of At0 from TLC plates. The volatilization of At0 subsequentially leads to a decrease in the amounts of AtO3−(V) and At−(−I) on the TLC plates in dynamical equilibria.
Such formation mechanism of volatile astatine species At0(0) through the oxidation–reduction reaction between AtO3−(V) and At−(−I) in dynamical equilibria could provide an account of distillation of astatine solutions under reduction conditions (0.05 M FeSO4 in 0.5 M H2SO4 [2] and 0.75 M FeSO4 in 1.5 M H2SO4 [13]).
Conclusions
The astatine radionuclides 209,210,211At produced in the 7Li induced reaction of 209Bi were used to fundamental experimental studies on chemical properties of astatine by TLC: oxidation–reduction between the dissolved astatine species, AtO4−, AtO3− and At−, as well as formation of the volatile species of At0.
The component analysis of relative amounts of the astatine species was carried out for astatine aqueous solutions of 6 × 10−6–6 × 10−3 mol L−1 KIO4, 1 × 10−4–1 × 10−1 mol L−1 Na2SO3 and 9.1–28.6% NH2NH2·H2O. The oxidizing and reducing reagents varied the composition profiles of relative amounts of the astatine species. The loss of astatine activities from TLC plates for aqueous solutions with/without KIO4 or Na2SO3 was estimated to be less than approximately 20% of the total activities used to TLC plates. In contrast, the presence of hydrazine causes the remarkable loss of astatine activities, 24–50%.
This work confirmed that At0(0) characterized high volatility formed in the oxidation–reduction reaction between At−(−I) and AtO3−(V) in dynamic equilibria and volatilized from TLC plates in the development as suggested in our previous study in Ref. [14]. The TLC analysis on a silica gel should not be conducted without adequate ventilation. The volatile species At0 involves risk regarding radiation safety.
The formation of At0 provides not only greater risk but also decided uncertainty in TLC analysis for astatine solutions including strong reducing reagents. The studies on high performance liquid chromatography (HPLC) are required to establish a sophisticated analytical method of the astatine anions in solutions as a complementary method of TLC. However, it should be noted that TLC is a simple and conventional analytical method of the astatine anions.
References
Wilbur DS (2013) Enigmatic astatine. Nat Chem 5:246
Johnson GL, Leininger RF, Segre E (1949) Chemical properties of astatine. I. J Chem Phys 17:1–10
Appelman EH (1960) The radiochemistry of astatine. Natl Acad Sci. Natlional Research Council, Nuclear Science Series, U S Atomic Energy Commission
Rössler K, Tornau W, Stöcklin G (1974) Rapid separation of carrier-free inorganic and organic compounds of radioiodine and astatine by high-pressure liquid chromatograpy. J Radioanal Nucl Chem 21:199–209
Dreyer I, Dreyer R, Norseev YV, Chalkin VA (1978) Synthese anorganischer astatverbindungen und untersuchung ihrer eigenschaften mittels papierelektrophorese und paierchromatographie. Radiochem Radioanal Lett 33:291–300 [in German]
Visser GWM, Diemer EL (1983) Inorganic astatine chemistry: formation of complexes of astatine. Radiochim Acta 33:145–151
Milanov M, Deberenz V, Khalkin VA, Marinov A (1984) Chemical properties of positive singly charged astatine ion in aqueous solution. J Radioanal Nucl Chem 83:291–299
Dreyer R, Dreyer I, Fischer S, Hartmann H, Rösch F (1985) Synthesis and characterization of cationic astatine compounds with sulpher-containing ligands stable in aqueous solutions. J Radioanal Nucl Chem 96:333–342
Ludwig R, Fischer S, Hussein H, Frind M, Dreyer R (1989) Stability constants of At(I)-complexes with thiourea, iodide and mixed ligands in ethanol and water. J Radioanal Nucl Chem 134:141–149
Champion J, Alliot C, Huclier S, Deniaud D, Asfari Z, Montavon G (2009) Determination of stability constants between complexing agents and At(I) and At(III) species present at ultra-trace concentrations. Inorg Chim Acta 362:2654–2661
Champion J, Alliot C, Renault E, Mokili BM, Chérel M, Galland N, Montavon G (2010) Astatine standard redox potentials and speciation in acidic medium. J Phys Chem A 114:576–582
Campion J, Sabatié-Gogova A, Bassal F, Ayed T, Alliot C, Gallland N, Montavon G (2013) Investigation of astatine(III) hydrolyzed species: experiments and relativistic calculations. J Phys Chem A 117:1983–1990
Balkin EB, Hamlin DK, Gagnon K, Chyan MK, Pal S, Watanabe S, Wilber DS (2013) Evaluation of a wet chemistry method for isolation of cyclotron produced [211At]astatine. Appl Sci 3:636–655
Nishinaka I, Hashimoto K, Suzuki H (2018) Thin layer chromatography for astatine and iodine in solutions prepared by dry distillation. J Radioanal Nucl Chem 318:897–905
Nishinaka I, Yokoyama A, Washiyama K, Maeda E, Watanabe S, Hashimoto K, Ishioka NS, Makii H, Toyoshima A, Yamada N, Amano R (2015) Production and separation of astatine isotopes in the 7Li + natPb reaction. J Radioanal Nucl Chem 304:1077–1083
Yordanov AT, Pozzi O, Carlin S, Akabani G, Wieland B, Zalutsky MR (2004) Wet harvesting of no-carrier-added 211At from an irradiated 209Bi target for radiopharmaceutical applications. J Radioanal Nucl Chem 262:593–599
Acknowledgements
The authors thank the crew of the JAEA Tandem Accelerator for accelerator operation. We are thankful to Dr. M. Asai for his help for γ-ray and α-ray spectroscopies and H. Saeki for visualizing imaging plates by BAS. This work was supported by JSPS KAKENHI Grant Numbers JP2360013, JP15K04741 and JP18K11939.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishinaka, I., Hashimoto, K. & Suzuki, H. Speciation of astatine reacted with oxidizing and reducing reagents by thin layer chromatography: formation of volatile astatine. J Radioanal Nucl Chem 322, 2003–2009 (2019). https://doi.org/10.1007/s10967-019-06900-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06900-3