Abstract
The through-diffusion method and batch sorption experiments were used to explore the influence of pH on the diffusion behavior of 75Se(IV) in matrix Beishan granite (BsG). In the pH range of 2.0–8.5, the De values of 75Se(IV) in BsG decreased first and then increased with pH increasing, while the changing trend of Kd was nearly opposite. It was speculated that the influence of pH on the diffusion of 75Se(IV) in BsG was due to the joint effects of different species distribution of Se, change in surface charge of BsG and change in ionic strength at various pH values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
So far, the deep geological disposal, which depends on a multi-barrier system, is internationally accepted to be the most effective way for the safety disposal of high-level radioactive wastes (HLW) [1]. The multi-barrier system is typically composed of an engineered barrier and the natural geological barrier provided by the host rock and its surroundings, to isolate the wastes from the accessible environment [2]. To make sure the wastes can be safely isolated in a long time scale which is needed for the HLW radioactivity decay to the natural level, the pre-safety assessment of the deep geological repository is an essential task [3]. While host rocks, as the final barrier in the HLW disposal repository system, provide effective retention for radionuclides presented in the groundwater flow [4, 5]. Consequently, the investigation on the migration behaviors of key radionuclides in host rocks of the repository site is an important aspect concerned in the pre-safety assessment of the repository [6]. Meanwhile, crystalline rocks, such as granite, have been considered as potential host rocks of the deep geological repositories in many countries. In China, Beishan granite (BsG), at Beishan area in northwest China’s Gansu Province, is considered as a candidate host rock for China’s potential deep geological repository [7].
As a fission product presented in spent nuclear fuel, 79Se is identified as one of the largest contributors [8] for the performance assessment of HLW disposal and one of the critical radionuclides highly concerned for the long-term safety of geological repositories due to its long half-life (~ 2.95 × 105 year) [9], chemical and radiological toxicity and high mobility [10]. Meanwhile, as a redox-sensitive nuclide, the solubility and mobility of 79Se highly depend on its oxidation states and chemical form which are significantly influenced by the surrounding Eh–pH conditions [11, 12]. Anionic Se(IV) and Se(VI) are more soluble and prefer to diffuse, while selenium with lower oxidation states (0, -I and -II) are usually present in solid form and possess low solubility [13]. To obtain parameters and develop reliable models for the pre-safety assessment of China’s potential repository, it is quite necessary to study the migration behavior of the dominant aqueous species of 79Se in Beishan granite, and the choice of anionic 79Se(IV), which is a more mobile species of selenium, for the investigation can provide more conservative results for the pre-safety assessment.
In the past, a certain amount of research work have been conducted in investigating the diffusion of selenium in natural minerals related to geologic disposal [8, 14,15,16,17,18,19,20,21], and most of which focused on the influence of Eh, T, I, mineral compositions and so on. However, the information for diffusion of Se(IV) in matrix granite is still limited, and even few work have paid attention to the influence of pH. Specially, the pH values of the groundwater maybe change with different rocks it passes through. Besides, the dissolution of the natural minerals and the change in the concentration of CO2 in groundwater may also contribute to the change of pH in groundwater. Consequently, density of the positively or negatively charged sites of natural minerals may be influenced by the change of pH values, and the corrosion of the natural minerals caused by the groundwater may also be affected by the pH difference in turn [22].
Need to be mentioned that the diffusion experiments conducted with relatively high selenium concentrations [16, 17, 23] (approximately mmol/L magnitude) may cause an overestimation or an inadequate mechanism of diffusion, and conduct of diffusion experiments with selenium concentrations close to realistic situations of the geologic repository is crucial to understand the real migration behaviors of selenium in granite [20].
Radionuclide diffusion is closely related to the pore water chemistry, and pH is an important factor in this process [23]. With groundwater as a major carrier for migration, the species distribution of redox-sensitive Se may be changed by the difference in the groundwater pH values, which may have significant influence on their migration behaviors. As far as we know, studies focused on the influence of pH conditions on Se(IV) diffusion behaviors in matrix Beishan granite have not been published previously. The objective of this work was to systematically investigate the diffusion behaviors of 79Se(IV) in matrix Beishan granite at different pH values for obtaining reliable parameters and exploring the diffusion and migration mechanisms of Se in the granitic rock. The diffusion behaviors of 75Se(IV) (T1/2 = 120 d, as a surrogate of Se-79) in BsG slices were investigated by through-diffusion method in the pH range of 2.0–8.5 under aerobic conditions, while the sorption of 75Se(IV) was conduced by batch sorption experiments using crushed BsG. Furthermore, interface and species analysis were used to speculate possible mechanism related to the diffusion of 75Se(IV) in BsG under different pH conditions.
Experimental
Materials and characterization
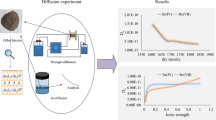
The granite core sample used in this work was drilled out at about 600 m depth at Beishan area, Gansu Province, China. The Beishan granite (BsG) slices used for the through-diffusion experiments were cut from the core sample, which were 64.0 ± 0.5 mm in diameter and 5.0 ± 0.3 mm in thickness. While the BsG powder used for the batch sorption experiments and characterization were crushed from the same core sample and sieved through a 200 mesh sieve. The morphologies of the BsG samples were shown in Fig. 1.
The BsG slices were air-dried for the measurement of dry density (ρ), and porosity (ε) were gained by water immersion technique [24], both data are shown in Table 1, together with the experimental conditions in the diffusion experiments. Mineralogical compositions of BsG were accessed by XRD method (Table 2), showing that the main mineralogical compositions are quartz, albite, microcline and biotite. While the chemical and elemental compositions of BsG were shown in Table 3, which were characterized by the X-ray fluorescence spectrometry (XRFS) (ARL ADVANT XP + , Thermo electron co.) and X-ray photoelectron spectroscopy (XPS) (Axis Ultra).
The carrier-free Na75SeO3 in 0.1 mol/L HCl used for the through-diffusion and batch sorption experiments was purchased from Eckert & Ziegler (USA). All other reagents were in analytical grade.
Through-diffusion experiments
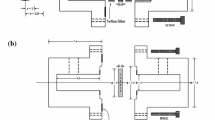
The through-diffusion experiments were conducted by the diffusion cells which had been described detailedly in our previous work [25, 26]. Figure 2 schematically represents the diffusion cell, which consists of a larger source cell (~ 1800 mL) and a smaller sampling cell (~ 70 mL).
The BsG slice was mounted between the two cells and was strictly sealed to make sure BsG slice was the only diffusion path for 75Se(IV), then 0.1 M NaClO4 solution with different pH values, both as background solution and to control the ionic strength, was added into both cells and kept at the same liquid-level, and the pH values for each cell were adjusted by addition of negligible volume of HClO4 or NaOH solution if necessary. The temperature was controlled by the thermo-tanks with a temperature precision of ± 0.5 °C. After pre-equilibrium for 30 days, negligible volume of radioactive 75SeO32− solution (with no additional stable isotope carrier) was injected to the source cell and a concentration at the magnitude of about 10−9 mol/L for 75Se(IV) was acquired. Then the analysis of sample was conducted by taking 1.0 mL solution from the sampling cell and detected for the radioactivity by a gamma radiation detector (PerkinElmer 2470) regularly. Meanwhile, 1.0 mL 0.1 M NaClO4 solution was injected into the cell after each sampling to maintain a constant liquid-level.
Thereafter sampling analysis was conducted periodically in the same way, at a 2-day interval for the first 2 months and a 7-day interval for the rest with a total of 210-day. The pH and Eh values were monitored by the pH meter and potentiometers regularly throughout the experimental period.
Model description
The experimental data gained from the diffusion experiments were fitted by DKFIT program, which was written to optimize parameters of the effective diffusion coefficient (De) and adsorption distribution coefficient (Kd) from “through-diffusion” experiments, based on the Fick’s first law, as described in our previous works [25, 27, 28].
According to the setup of our experiments, the volume of the source cell (Vin ≈ 1800 mL) is much larger than the volume of the sampling cell (Vout ≈ 50 mL), thus it can be considered that the concentration of 75Se(IV) in the source cell is kept constant, while the concentration of 75Se(IV) in the sampling cell increases, as the experiment proceeds. Therefore, the diffusion of 75Se(IV) in the BsG could be approximately described by the constant inlet concentration-increasing outlet concentration through-diffusion model (CC-IC) [25, 27, 28] and can be described by the one-dimensional diffusion equation:
where C(x,t) (cpm/mL) is the 75Se(IV) concentration in the solution at time t (s) and diffusion distance x (m) along the diffusion direction in BsG slice; De (m2/s) is the effective diffusion coefficient of 75Se in the BsG; ε (−) and ρ (kg/dm3) are porosity and dry density of the BsG slice, respectively; λ (s−1) is the decay constant of 75Se; S(x,t) (cpm/g) represents the sorbed 75Se concentration on BsG at time t and position x. S(x,t) was described by a linear adsorption model:
Then Eq. (1) can be transformed into:
According to the experiments, the initial and boundary conditions for Eq. (4) are given by:
where L (m) and A (m2) are thickness and effective diffusion area of BsG slice; Vu (m3) is the volume of the source cell and Vd (m3) is the volume of the sampling cell; C0 (cpm/mL) is the initial concentration of 75Se in the source cell. Cu(t) (cpm/mL) and Cd(t) (cpm/mL) are the concentration of 75Se in the source and sampling cell when the experiment lasts for time t (s), equally to C(0,t) and C(L,t) in value, respectively. In our experiment, Vu ≫ Vd, so the decrease of Cu can be ignored.
To obtain De, the experimental data were fitted with the diffusion Eq. (4) by a finite difference scheme, based on the following assumptions:
Then Eq. (4) is transformed into:
when Δt and Δx are chosen to satisfy the condition descript in Eq. (9) (as shown in the following), Eq. (8) will have a stable numerical solution:
In each experiment, the values of De and Kd could be obtained by fitting the breakthrough curve of Cd(t)/C0 as a function of t using the program DKFIT.
Batch sorption experiments
The sorption experiments were conducted in 10 mL polyethylene tubes, without adding stable SeO32− carriers, under aerobic condition at 26 °C, to better understand the migration behavior of Se(IV) diffusion in BsG. To be consistent with the diffusion experiments, the stock suspension of BsG (m/V = 20 g/L) was prepared in 0.1 M NaClO4 solution, and negligible volumes of 0.01–0.1 M HClO4 or NaOH solutions were used to adjusted the pH values of the system. After pre-equilibrium, the radioactive Na 752 SeO3 solution (negligible volume) was added into the tubes to obtain a concentration of Se(IV) at the magnitude of about 10−9 mol/L, which is in the same order of magnitude with Se(IV) concentration in diffusion experiments. Then after equilibrated for 5 days, 1.0 mL of both the suspension and the supernatant obtained by phase separation (filtration with 0.22 μm polypropylene membrane) were measured by Gamma radiation detector (Perkin Elmer 2470) to determine the radioactivity of 75Se, separately.
Then the distribution coefficient (Kd, L/kg) can be calculated by the following expression:
where A0 (cpm/mL) is the total activity of 1.0 mL suspension, Aeq (cpm/mL) is the activity of 1.0 mL supernatant, V (L) is the volume of the total suspension, while m (kg) is the mass of the BsG powders.
Titration procedure of BsG
Potentiometric titration of BsG powder was performed to analyze the acid–base properties of BsG surface using a Mettler Toledo T70 Automatic Titrimeter at room temperature under a nitrogen atmosphere. The pH values were measured by a combined glass electrode and before the titration, the electrode was calibrated by three standard buffer solutions of pH 4.01, 6.86 and 9.18. The BsG suspension, with 0.1 mol/L NaClO4 as background electrolyte, was purged with nitrogen for about 8 h first to reach equilibrium, then was titrated with a CO2-free NaOH (0.096 mol/L) solution to pH 11, and further titrated with a CO2-free HClO4 (0.103 mol/L) solution until the pH reached 3. The blank sample, with equal volume of 0.1 mol/L NaClO4 solution without BsG powder, was titrated by the same way as well. The point of zero charge pHpzc and surface proton excess △Q (mol g−1) were obtained using the method introduced by Tertre et al. [29].
XAS analysis for selenium speciation
The X-ray absorption spectroscopy (XAS) was mainly used to analyze the dominated species of selenium on BsG. The BsG suspension was prepared with a S/L ratio of 20 g/L and 0.1 mmol/L Se(IV) under different needed pH values. After equilibration for 5 days, the BsG powder samples under needed pH values were dried and collected, together with the reference compounds of Se(0), Na2SeO3 and Na2SeO4, analyzed at the Se K-edge (12 658 eV) on beamline 1W2B of Beijing Synchrotron Radiation Facility (Beijing, China). The Se(0) foil reference was measured for the accurate energy calibration, while the BsG samples were measured in fluorescence mode and the reference data were collected in transmission mode. A linear combination fit (LCF) was used to X-ray absorption near edge structure (XANES) spectra to gain the samples speciation information. The LCF was performed using the IFEFFIT package [30].
Results and discussion
The effective diffusion coefficients (D e) and adsorption distribution coefficients (Kd) of 75Se(IV) in BsG
Figure 3 shows the fitted results of C(t)/C0 versus time for 75Se(IV) diffusion in matrix BsG by DKFIT, combined with the values of correlation coefficient R2 which were in the range of 0.91–0.98 (Table 4). The effective diffusion coefficients (De) and adsorption distribution coefficients (Kd) of 75Se(IV) in BsG obtained from the diffusion experiments fitted by DKFIT were listed in Table 4, showing that De values of 75Se(IV) diffusion in BsG are in the range of (0.86–4.81) × 10−13 m2/s, and Kd values are in the range of (0.59–2.46) × 10−13 mL/g, when the pH varied from 2.0 to 8.5. Meanwhile, the De values under different pH conditions obtained in this work are comparable to De values of different types of granite reported in the literature, including both the matrix and crushed granite, as listed in Table 5, showing that the results derived from the matrix BsG here are in good agreement with literature data [17, 20] in magnitude, while the De values obtained from the crushed granite [15, 18] are significantly larger than the ones derived from the matrix granite.
Moreover, several Kd values derived from the batch sorption experiments were also shown in Table 4, indicating that the Kd values obtained from the sorption experiments were remarkably larger than the ones derived from the diffusion experiments, which was possibly due to the fact that, the particle size of BsG powder sused in the sorption experiments was much smaller than the BsG slices used in the diffusion experiments, and the remarkable smaller particle size and larger surface area of BsG powders would significantly promoted the sorption of Se onto BsG [31, 32].
The pH and Eh values of each diffusion cell did not change significantly throughout the experiment periods, thus only final values of pH and Eh are displayed. Furthermore, no solid phase can be observed in the diffusion cells.
Effects of pH values on the diffusion and sorption of 75Se(IV) in BsG
The relationship between De and Kd values of 75Se(IV) diffusion and sorption in BsG as a function of pH were presented in Figs. 4 and 5, showing that De values decreased first and then increased, while Kd values decreased first and then increased and decreased again, with pH increased from 2.0 to 8.5, and it seemed that there is a shift or anticorrelation in the relationship between the De and Kd values to some extent, where both have an extreme value at pH 6, except for the point in pH 2.0. It should be noticed that the changing trend of Kd values of 75Se(IV) in BsG derived from the diffusion experiments was in consistent with the ones obtained from the sorption experiments, though the numerical results differ greatly, as shown in Fig. 5.
Meanwhile, the sorption results of 75Se(IV) in BsG by the batch sorption experiments in the whole pH range of 1.0–11.0 was presented in Fig. 6. It indicated that the total sorption curve can be divided into three parts, with pH 3.5 and pH 5.5 as the cut-off point, showing that the sorption of 75Se(IV) in BsG decreased first, then increased, and decreased again with the increase of pH values. It is thus clear that the five chosen pH points investigated in the diffusion experiments can basically reflect the general sorption behaviors of 75Se(IV) in BsG, and from this perspective, the diffusion experiments under the chosen pH conditions may basically reflect the diffusion behaviors of 75Se(IV) in BsG in the whole pH range as well.
In our previous work [18], the migration of 75Se(IV) in crushed BsG using the in-diffusion capillary method with stable isotope carrier (Cse ~ 10−6 mol/L) had been investigated and it was found that, the diffusion of 75Se(IV) in crushed BsG decreased first and then increased in the pH range of 2–10, with the slowest 75Se(IV) diffusion occurred around pH 5, while the sorption results is inversely proportional to the diffusion ones. It was speculated that the sorption of 75Se(IV) in BsG under various pH conditions was mainly dominated by the different surface charging reactions and co-impact of potential Se(IV) sorbents such as mineral phase containing various metal elements (Al, Fe, Mg, Ca, etc.). Moreover, the changing trend of De values is almost in consistent with the results here, though the De values is about one order of magnitude greater than those obtained here, because crushed BsG was used in our previous work. Apart from this, the Kd values in our previous work were a little smaller than those here, and showed a certain degree of variety in the changing trend as well, maybe it was due to the variety in the Se(IV) concentration (no additional stable isotope carrier was used here), since the behaviors of nuclides at very low concentration may be different from the behaviors at regular concentration.
Possible mechanisms for 75Se(IV) diffusion in BsG
Intuitively, the difference in 75Se(IV) diffusion at different pH conditions seemed to be caused by the diversity in sorption results, since the Kd values are nearly inversely proportional to the De values. To explore the possible diffusion mechanism of Se(IV) in matrix BsG under various pH conditions from the perspective of the diffusion behavior itself, the results were analyzed focusing on three aspects: the objective nuclide Se, the diffusion medium BsG and the diffusion solution system in which the diffusion process would carry out.
Firstly, the surfaces of BsG slices used in the diffusion experiments of both the source cell and the sampling cell side were analyzed by XPS after the diffusion experiments, to clarify the dissolution situation of BsG under different pH conditions during the diffusion process, as shown in Table 6 and Fig. 7. It indicated that there existed a certain degree of mineral dissolution, and the dissolution extent of each element was different, while the dissolution of Fe and Ca is relatively obvious. Meanwhile, the dissolved amount of Fe and Ca increased with pH decreased.
Meanwhile, the diffusion solution of both the source cell and sampling cell were analyzed by ICP (Inductive Coupled Plasma Emission Spectrometer) after the diffusion experiments were finished, to confirm the composition of the diffusion solution under different pH conditions in the end of the diffusion experiments, as presented in Table 7 and Fig. 8. As can be seen from Fig. 8, the concentration of Fe, Ca, Mg increased with pH decreased, which was mainly caused by the dissolution of BsG due to the more acidic solution when pH decreased and the long experimental period. The results for Fe and Ca were in consistent with the the results of XPS analysis, and the dissolved metal ions would contribute to the whole ionic strength of the solution.
At the same time, in order to confirm that whether the ions introduced into the diffusion solution due to the mineral dissolution of BsG under different pH conditions, especially the elements such as Mg, Fe, Ca which are susceptible to precipitation or redox with Se, would cause precipitation or redox reaction of Se in the solution and further affect its diffusion, the precipitation equilibrium constant Ksp of Se and related elements in solution and redox reactions between Se(IV) and Fe2+ were calculated. When conducting related calculations, the concentration of each relevant element (such as Mg, Fe, Ca) was determined by the maximum concentration detected by ICP method (as shown in Table 7), while the original concentration of SeO32− in source cell (at the magnitude of 10−9 mol/L) before diffusion was adopted, to ensure that the most conservative calculation results were obtained.
For the precipitation calculation, the concentration product (Qc) of possible precipitation reactions were calculated and compared to corresponding solubility product (K Ɵsp ), where lgK Ɵsp (CaSeO3) = − (6.40 ± 0.25), lgK Ɵsp (MgSeO3) = − (5.82 ± 0.25). The calculations showed that Qc < K Ɵsp for both possible precipitation reactions, thus the precipitation reactions would not occur, that is the metal ions produced by the BsG dissolution would not cause the precipitation of Se in the solution. The calculation results are as follows:
To estimate whether redox reactions between Se(IV) and Fe2+ would occur from the perspective of thermodynamics, the ∆rG (Gibbs free energy) of related redox reactions were calculated using the most recently reported thermodynamic data [33] and the theoretical pH values at which each reaction can occur were obtained, as listed below each reaction formula. It was found that when the maximum dissolved concentration of Fe detected at pH 2 (81 ppm, as shown in Table 7) were used in the redox reaction calculation, only when theoretical pH value was higher than 2.65 the redox reaction may occur, and this pH value is larger than 2, so the Se(IV) would not be reduced to Se(0), and the calculation results are as follows:
Therefore, the dissolution of BsG slice would just increase the concentration of parts of the metal ions in the solution, and neither precipitation nor redox reactions of Se would happen due to the relatively low dissolved concentrations of metal ions.
Secondly, the XANES spectra of BsG powder interacting with Se(IV) under different pH conditions varied from 2 to 8.5 are shown in Fig. 9, along with reference spectra of Se(0), Se(IV) and Se(VI), showing that all the samples under investigated pH values presented the same energy peak with Se(IV) at 12,664 eV. Therefore, the dominated species of selenium under different investigated pH values was still Se(IV), and no redox reaction had taken place.
Meanwhile, the monitored pH and Eh values during the experiments were superimposed in the theoretical Eh–pH diagram calculated by PHREEQC to validate the dominated species of selenium under different pH conditions (Fig. 10). It indicated that the dominated species of selenium were H2SeO3, HSeO3− and SeO32− with pH increased from 2.0 to 8.5, and Se(IV) was still the dominating oxidation state in the whole pH range, which was in accordance with the results obtained from the XAS analysis.
Experimental data are superimposed on diagram marked as red dots.
Furthermore, based on the pH and Eh values measured during the experiments, the more detailed species distribution of Se at different pH values were obtained by the calculation of CHEMSPEC [34], and the abstraction of average charge distribution (electric charge per Se atom) of single Se atom was further gained to have a more quantitative understanding for the charged conditions of selenium, as shown in Fig. 11. It can be seen that in the investigated pH range, the dominated selenium species was Se(IV), which are in accord with both the XAS and superimposed Eh–pH diagram results, while there is a significant change in the abstraction of the quantitative charge per Se atom, with the change of pH values. Meanwhile, the surface proton excess △Q (mol/g) of BsG with pH from the titration results was shown in Fig. 12, indicating that the pHpzc of BsG was about 5.6. Thus, combined with the surface proton excess of BsG surface and the species distribution of selenium, it was speculated that the diffusion phenomena at different pH conditions can be illustrated from two phases, one phase is when the pH is in the range of 5.6–8.5, while the other phase is when the pH is in the range of 2–5.6.
At the first phase, when the pH is higher than 5.6 (pHpzc), both the surface of BsG and Se were negatively charged, and the electronegativity of both increased with pH increasing, thus the anion repulsion effect was enhanced [35], showing that the diffusion increased with pH increasing.
At the second phase, when the pH is lower than 5.6, the surface of BsG was positively charged, while the Se was negatively charged. Moreover, the positive charge of BsG decreased with pH increased, whereas the negative charge of Se increased with pH increased, showing a negative correlation between each other on the whole.
Furthermore, based on the species distribution of Se at different pH values derived from the superimposed Eh–pH diagram and calculation of CHEMSPEC, the diffusion of Se in the pH range less than 6 was analyzed in detail and it was found that: At pH 2, the uncharged H2SeO3 was the predominant species of Se, and there would be almost no electrostatic interaction between BsG and Se, so the diffusion process should be dominated by the “free diffusion”. While at pH 4, the negatively charged HSeO3− was the dominated species, together with the weak positively charged BsG surface, there would be weak electrostatic attraction interaction between BsG and Se, and the diffusion would be dominated by the “weak dragged diffusion”. Thus, the De values decreased with pH increased in the pH range of 2–4, as shown in Table 4 and Fig. 4. Then, when the pH increased to 6, the negatively charged HSeO3− was still the predominant species, while the surface of BsG was also weak negatively charged, there would be weak electrostatic repulsion interaction between BsG and Se, so the “weak anion repulsion” effects would begin to play a role (Fig. 13). It should also be noted that, when the pH decreased to certain degree, the change in the ionic strength caused by the adjustment of pH values and mineral dissolution of BsG should not be ignored. As a consequence, under the joint effects of both the mineral dissolution and pH adjustment, the ionic strength of the solution would increase with pH decreased when pH was lower than 6. With the ionic strength of the solution increased, the electrode double layers on the surface of BsG’s diffusion pores, which were formed by the electrostatic interaction between charged surface of the diffusion pores and the background electrode solution, would be shielded more effectively and compressed, thus accelerating the diffusion of Se in BsG [35].
On the whole, it was speculated that the change in the ionic strength and the species distribution of Se were the main reasons for the increased De values with pH decreased when the pH is lower than 6. While the increased diffusion of Se with increased pH when the pH values was larger than 6 was due to the increased electronegativity of both the surface of BsG and Se with higher pH values.
Nevertheless, the use of radionuclide 75Se and its extremely low concentration in the diffusion and sorption experiments severely limited the spectroscopic and microstructure analysis of the samples (especially BsG slices and diffusion solutions, etc.) under different pH conditions. As a consequence, more further exploration and microstructural information are required in order to estimate the microscopic diffusion mechanism for Se(IV) in BsG at different pH conditions, while the development of in situ microstructure analysis methods for nuclides at very low concentrations will be of great help to study the diffusion mechanism of key nuclides in related media close to the actual repository environment and concentration conditions.
Conclusion
This study was conducted using the through-diffusion experimental method, combined with batch sorption experiments, samples characterization and species analysis, to investigate the influence of pH on the diffusion behavior of 75Se(IV) in matrix BsG. The diffusion and sorption behavior of 75Se(IV) in BsG was significantly affected by pH in the investigated pH range of 2.0–8.5, showing that the De values of 75Se(IV) in BsG decreased first and then increased with increase of pH values, while the changing trend of Kd values was nearly the opposite, with both parameters had extreme values at pH 6. There existed a certain degree of mineral dissolution in the surface of BsG slices, especially at lower pH conditions, but the mineral dissolution would introduce neither precipitation nor redox reaction of 75Se(IV), except for increasing parts of the ions’ concentrations in the solution. It was believed that the influence of pH on the diffusion of 75Se(IV) in BsG was the comprehensive effects of different species distribution of Se, the variety in the charges of BsG surface and the change in the ionic strength at various pH values.
The significant influence of pH on the diffusion and sorption behavior of Se(IV) in BsG indicating that in the process for evaluating the migration behavior of key nuclides for the pre-safety assessment of the potential repository, the effects of pH factors should be fully considered and the real pH conditions of the repository should be clarified to obtain migration parameters that are closer to the actual repository conditions as much as possible.
References
Ewing RC (1999) Radioactive waste: less geology in the geological disposal of nuclear waste. Science 286(5439):415–417
Soler JM, Landa J, Havlova V, Tachi Y, Ebina T, Sardini P, Siitari-Kauppi M, Eikenberg J, Martin AJ (2015) Comparative modeling of an in situ diffusion experiment in granite at the Grimsel Test Site. J Contam Hydrol 179:89–101
Chapman N, Hooper A (2012) The disposal of radioactive wastes underground. Proc Geol Assoc 123(1):46–63
Agbogun HMD, Al TA, Hussein EMA (2013) Three dimensional imaging of porosity and tracer concentration distributions in a dolostone sample during diffusion experiments using X-ray micro-CT. J Contam Hydrol 145(1):44–53
Poteri A, Nordman H, Pulkkanen V-M, Smith P (2014) Radionuclide Transport in the Repository Near-Field and Far-Field. POSIVA Report 2014-2: 17-24. Posiva Oy. www.posiva.fi/files/3516/POSIVA_2014-02.pdf
Grambow B (2008) Mobile fission and activation products in nuclear waste disposal. J Contam Hydrol 102(3):180–186
Wang J (2010) High-level radioactive waste disposal in China: update 2010. J Rock Mech Geotech Eng 2(1):1–11
Descostes M, Blin V, Bazer-Bachi F, Meier P, Grenut B, Radwan J, Schlegel ML, Buschaert S, Coelho D, Tevissen E (2008) Diffusion of anionic species in Callovo-Oxfordian argillites and Oxfordian limestones (Meuse/Haute–Marne, France). Appl Geochem 23(4):655–677
Fernández-Martínez A, Charlet L (2009) Selenium environmental cycling and bioavailability: a structural chemist point of view. Rev Environ Sci Biotechnol 8:81–110
Hjerpe T, Ikonen ATK, Broed R (2010) Biosphere Assessment Report 2009. POSIVA Report 2010-3: 36-38. Posiva Oy. www.posiva.fi/files/1230/POSIVA_2010-03web.pdf
Montavon G, Guo Z, Lützenkirchen J, Alhajji E, Kedziorek MAM, Bourg ACM, Grambow B (2009) Interaction of selenite with MX-80 bentonite: effect of minor phases, pH, selenite loading, solution composition and compaction. Colloids Surf A 332(2–3):71–77
Charlet L, Kang M, Bardelli F, Kirsch R, Géhin A, Grenèche J-M, Chen F (2012) Nanocomposite pyrite–greigite reactivity toward Se(IV)/Se(VI). Environ Sci Technol 46(9):4869–4876
Scheinost AC, Charlet L (2008) Selenite reduction by mackinawite, magnetite and siderite: XAS characterization of nanosized redox products. Environ Sci Technol 42(6):1984–1989
Sato H, Miyamoto S (2004) Diffusion behaviour of selenite and hydroselenide in compacted bentonite. Appl Clay Sci 26(1):47–55
Tsai T-L, Lee C-P, Lin T-Y, Wei H-J, Men L-C (2010) Evaluation of sorption and diffusion behavior of selenium in crushed granite by through-diffusion column tests. J Radioanal Nucl Chem 285(3):733–739
Wu T, Wang H, Zheng Q, Zhao YL, Van Loon LR (2014) Diffusion behavior of Se(IV) and Re(VII) in GMZ bentonite. Appl Clay Sci 101:136–140
Ikonen J, Voutilainen M, Söderlund M, Jokelainen L, Siitari-Kauppi M, Martin A (2016) Sorption and diffusion of selenium oxyanions in granitic rock. J Contam Hydrol 192:203–211
He J, Ma B, Kang M, Wang C, Nie Z, Liu C (2017) Migration of 75Se(IV) in crushed Beishan granite: effects of the iron content. J Hazard Mater 324:564–572
Wu T, Wang Z, Wang H, Zhang Z, Van Loon LR (2017) Salt effects on Re(VII) and Se(IV) diffusion in bentonite. Appl Clay Sci 141:104–110
Yang X, Ge X, He J, Wang C, Qi L, Wang X, Liu C (2018) Effects of mineral compositions on matrix diffusion and sorption of 75Se(IV) in granite. Environ Sci Technol 52(3):1320–1329
He J, Shi Y, Yang X, Zhou W, Li Y, Liu C (2018) Influence of Fe(II) on the Se(IV) sorption under oxic/anoxic conditions using bentonite. Chemosphere 193:376–384
Wersin P, Curti E, Appelo CAJ (2004) Modelling bentonite-water interactions at high solid/liquid ratios: swelling and diffuse double layer effects. Appl Clay Sci 26(1–4):249–257
Wang Z, Wang H, Li Q, Xu M, Guo Y, Li J, Wu T (2016) pH effect on Re(VII) and Se(IV) diffusion in compacted GMZ bentonite. Appl Geochem 73:1–7
Emerson DW (1990) Notes on mass properties of rocks density, porosity, permeability. Explor Geophys 21(4):209–216
Chen T, Sun M, Li C, Tian W, Liu X, Wang L, Wang X, Liu C (2010) The influence of temperature on the diffusion of 125I− in Beishan granite. Radiochim Acta 98(5):301–305
Li C, Liu XY, Chen T, Tian WY, Zheng Z, Wang LH, Liu CL (2012) The influence of pH on the sorption and diffusion of 99TcO4 − in Beishan granite. Radiochim Acta 100(7):449–455
Lu CJ, Liu CL, Chen T, Wang J, Wang XY, Su R, Sun JY, Yang RX, Zhang XS (2008) Determination of the effective diffusion coefficient for 125I− in Beishan granite. Radiochim Acta 96(2):111–117
Li C, Wang CL, Liu XY, Zheng Z, Wang LH, Zhu QQ, Kang ML, Chen T, Liu CL (2012) Effects of ionic strength and humic acid on 99TcO4 − sorption and diffusion in Beishan granite. J Radioanal Nucl Chem 293(3):751–756
Tertre E, Castet S, Berger G, Loubet M, Giffaut E (2006) Surface chemistry of kaolinite and Na-montmorillonite in aqueous electrolyte solutions at 25 and 60°C: experimental and modeling study. Geochim Cosmochim Acta 70(18):4579–4599
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12(4):537–541
Videnská K, Palágyi Š, Štamberg K, Vodiěková H, Havlová V (2013) Effect of grain size on the sorption and desorption of SeO4 2− and SeO3 2− in columns of crushed granite and fracture infill from granitic water under dynamic conditions. J Radioanal Nucl Chem 298(1):547–554
André M, Malmström ME, Neretnieks I (2009) Determination of sorption properties of intact rock samples: New methods based on electromigration. J Contam Hydrol 103(3–4):71–81
Olin Å, Noläng B, Osadchii EG, Öhman L-O, Rosén E (2004) Chemical thermodynamics of selenium. Elsevier, Uppsala
Zhu J, Wang X, Chen T, Liu C (2012) Chemical speciation code CHEMSPEC(C ++) and its applications. Sci Sin Chim 42(6):856
Van Loon LR, Glaus MA, Müller W (2007) Anion exclusion effects in compacted bentonites: towards a better understanding of anion diffusion. Appl Geochem 22(11):2536–2552
Acknowledgements
We thank the Special Foundation for High-level Radioactive Waste Disposal (2012-851) and the National Natural Science Foundation of China (NSFC, No. 11475008, U1530112, U1730245) for financial support. In addition, we are grateful to the 1W2B beamline at the Beijing Synchrotron Radiation Facility (Beijing, China) for providing beam time and assistance for the XAS measurements. The authors declare that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Yang, X., Wei, F. et al. The influence of pH on diffusion of 75Se(IV) in Beishan granite. J Radioanal Nucl Chem 319, 365–377 (2019). https://doi.org/10.1007/s10967-018-6344-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6344-9