Abstract
Reference materials are used worldwide and necessary for quality control purposes during analytical determinations. The present study describes the stability evaluation of a bovine kidney reference-material candidate. An isochronous layout was performed, in which the flasks involved are exposed at different temperatures for different time periods and then are analyzed at the same time at the end of the study. The mass fractions of ten inorganic constituents were determined using instrumental neutron activation analysis and inductively coupled plasma mass spectrometry. Statistical analysis, univariate and multivariate, showed no significant differences in composition between units exposed to the different temperatures and times. The reference material may be transported under normal transportation conditions and the certified values and uncertainties will continue to be valid for a period of 2 years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food of animal origin, particularly meat, should be part of a balanced diet, as they provide valuable nutrients that are beneficial to health. They are excellent sources of high quality protein, have iron and zinc readily available, vitamins B6, B12, B2 and vitamin A. They also increase the absorption of iron and zinc from vegetables. There is evidence of strong associations between the intake of food of animal origin and better physical and cognitive development in children [1].
Meat products have a great relevance in Brazil. Brazil is one of the main exporter countries of bovine meat in the world and the consumption of bovine meat per capita in Brazil is more than double the world average [2]. Meat production affects the economic development of the different producing regions and on the other hand, constitutes one of the most important sources of proteins and nutrients for the population. All these facts bring the need to perform different chemical measurements in meat, either to meet strict export standards, or local regulations for food safety and for quality control of products. In order to provide a tool for quality control of some of these chemical measurements, a new local reference material of bovine kidney has been prepared. This material was prepared using fresh bovine kidneys from cattle reared under controlled feeding conditions. The kidneys were grinded, lyophilized, sieved and sterilized with a gamma radiation dose of 10 kGy [3]. The material was identified as “IPEN RB1-Trace Elements in Bovine Kidney”.
The present work describes the short and long-term stability evaluation of this material using instrumental neutron activation analysis (INAA) and inductively coupled plasma mass spectrometry (ICP-MS) to cover a wider range of possible contaminants and nutrients.
Evaluation of the stability is an important step in the characterization process of any reference material. In the stability tests, the environmental conditions to which reference materials could be exposed during transport and storage are simulated to determine if there is significant degradation of the analytes or the matrix. They are generally based on measuring the analytes in different units of the reference material that have been exposed to different temperatures during different times. The short-term tests are used to decide whether the material is stable enough to become a reference material and to determine the conditions under which it should be transported to end users [4, 5]. They are usually performed for a short period of time (up to 8 weeks) and the reference materials are exposed to maximum temperatures which are estimated to occur during transportation. From the evaluation of the results, suitable conditions can be chosen to carry out transport in such a way that the instability is negligible. In this case, the uncertainty component due to short-term stability can be neglected [6].
Long-term tests are used to define the shelf life of the material. They are performed for a longer period, up to 2 years, at the reference material’s storage temperature [7].
In both stability tests, the existence of temporal trends at the different working temperatures should be analyzed using regression analysis. In the absence of trends, the measurements can be analyzed by analysis of variance (ANOVA) [6]. In order to reduce the analytical variability introduced by performing the measurements over a long period of time, an isochronous layout was chosen [4, 8]. In the isochronous layout all the units involved are analyzed at the same time under repeatable conditions at the end of the study.
In this study multivariate techniques such as principal component analysis (PCA) were applied to complement the traditional univariate analysis. Multivariate techniques can provide much more information than univariate techniques because they take into account the correlation among many variables at the same time. They make possible a graphical representation of a larger amount of information allowing a simpler visualization of the data set and facilitating its evaluation [9], showing underlying structures of data not visible by other means [10]. Despite their usefulness, there are not many precedents in using these statistical techniques in the characterization of reference materials. There are some antecedents in the literature for homogeneity assessment [3, 11,12,13,14] but only one for stability evaluation [15].
Finally, the uncertainty component due to long-term stability was estimated. In ISO Guide 35 [16] two approaches are recommended for the estimation of the uncertainty component due to long-term stability. The more conservative approach, used in this work, was proposed by Linsinger et al. [17].
Experimental
Experimental design
For the short-term stability evaluation of the bovine kidney reference-material candidate, nine bottles from the total batch of 175 were chosen using a random stratified scheme. Bottles were stored at 20 ± 3 °C (acclimatized room) and 50 ± 2 °C (Fanem Orion 515C oven) for different time periods (2, 4, 6 and 8 weeks). One bottle was stored at − 20 ± 2 °C (Brastemp Flex Freezer) to be used as control.
For the long-term stability evaluation, nine bottles from the total batch of 175 were also chosen using a random stratified scheme. Bottles were stored at 20 ± 3 °C (acclimatized room) for 3, 6, 9 and 12 months. This temperature was considered to be representative of the standard conditions in which reference materials are stored. One bottle was stored at − 20 ± 2 °C (Brastemp Flex Freezer) to be used as control.
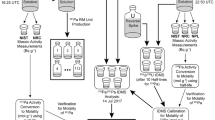
Figure 1 shows the isochronous layouts used. The shaded spaces represent the storage time of a given bottle at each temperature until the end of the study, when all measurements were performed. Three test portions of each bottle were randomly measured by INAA for the determination of Cl, Co, Fe, Mg, Mn, Na, Se and Zn and ICP-MS for the determination of As and Cu.
Element determination using INAA
Samples and elemental calibration standards preparation
Approximately 0.150 g of each sample was weighed in polyethylene bags, previously cleaned with 10% nitric acid and Milli-Q water, using a Shimadzu Libror AEL 40 SM analytical balance. Calibration standards were prepared from high purity standard solutions (LGC Standards, UK, and SPEX Certiprep Inc., USA) or appropriate dilutions of these standard solutions using Milli-Q water (Millipore Corporation, USA). Appropriate aliquots of these solutions were pipetted onto Whatman 40 filter papers and dried inside a laminar flow hood. After drying, filter papers were transferred to polyethylene bags, previously cleaned with 10% nitric acid and Milli-Q water.
Irradiation, measurement and interpretation of gamma spectrum
All the measured test portions were weighed in polyethylene bags. These bags were previously cleaned with 10% nitric acid and Milli-Q water. Test portions and element calibration standards were irradiated simultaneously under a thermal neutron flux of 4.6 × 1012 n cm−2 s−1 at the IEA-R1 nuclear research reactor of the Nuclear and Energy Research Institute, IPEN - CNEN/SP, São Paulo, Brazil.
Test portions and element calibration standards were irradiated for 20 s, to perform the determination of short lived radionuclides (27Mg, 56Mn and 38Cl). The samples and standards in sequence were measured for 300 s to determine 27Mg and 38Cl; and remeasured for 300 s to determine 56Mn after about 30 min decay period.
Test portions and element calibration standards were irradiated for 6 h to perform the determination of radionuclides with intermediate and long half lives (60Co, 59Fe, 24Na, 75Se and 65Zn). Sodium was measured for 1 h, after a 6-day decay period. Co, Fe, Se and Zn radionuclides were measured for 6 h, after a 21-day decay period.
Gamma ray measurements were performed using a GC2018 Canberra HPGe detector coupled to a Canberra DSA-1000 digital spectral analyzer. Gamma ray spectra were collected and processed using a Canberra Genie 2000 version 3.1 spectroscopy software and all element mass fraction calculations were carried out using a Microsoft Excel spreadsheet.
Element determination using ICP-MS
Samples and calibration standards preparation
About 0.200 g of each sample was weighted in MARSXpress tubes (CEM Corporation), previously decontaminated with 20% HNO3, using an Ohaus Adventurer AR2140 analytical balance. Samples were dissolved by acid digestion in a microwave furnace MARS 6 (CEM Corporation) using 10 ml of 20% (v/v) p.a. HNO3, high pressure and a maximum temperature of 200 °C. For the analytical determination of the elements, an eight-point multi-elemental calibration curve was prepared by appropriate dilution of high purity standard solutions (Perkin Elmer Pure Plus, USA, and SPEX Certiprep Inc., USA). All dilutions were made with 4% HNO3 as diluent. All used volumetric materials were gravimetrically calibrated before use.
Element determination
After digestion, sample solutions were cooled to room temperature and diluted with ultra-pure Milli-Q water to reach a 4% HNO3 concentration. Total determination of chemical elements was performed using an Agilent 7900 mass spectrometer (Hachioji, TY, Japan). Conditions used for the analysis are listed in Table 1.
Results and discussion
Tables 2 and 3 shows the mean value, expressed in mass fraction, for each element obtained in the bottles analyzed in the short-term and in the long-term stability studies, respectively. The uncertainty presented with each value in both tables is an expanded uncertainty, with a coverage factor k = 2 (approximately 95% confidence).
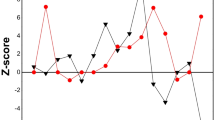
In order to evaluate the presence of trends, results obtained in both studies, at each analyzed temperature were plotted against time of the exposure. As an example, Fig. 2 presents the results obtained for analytes As and Zn. Linear regression fittings were performed, as recommended in ISO Guide 35 [16]. Then, ANOVA for the linear regression was performed. This analysis allows evaluating if there is any significant slope, and therefore a trend, in results. The results obtained are shown in Tables 4 and 5.
As it can be seen in Table 4 and Table 5, F < Fc and p < 0.05 for all analyzed elements at both stability studies showing the absence of any significant trend at a 95% confidence level.
For the short-term stability study, after discarding the presence of trends, a one-way analysis of variance was performed to evaluate if there were significant differences between the two studied temperatures. Table 6 presents the obtained values for F calculated and p values, along with mean square values (MS), obtained by ANOVA for each analyzed element. In all cases the obtained results were satisfactory, since no statistically significant differences were observed between bottles exposed at each temperature.
To confirm and complement this univariate analysis, a principal component analysis was performed for the short-term study using Statistica 7.0 software program [18]. Figure 3 shows the score plots for the first two principal components which explain approximately 60% of the total variability. Bottles analyzed at different temperatures are represented by a different type of mark. Results of all the analyzed test portions are shown in the graph, so each bottle appears three times. As it may be seen, no evident group or tendency can be observed, indicating the equivalence of the samples in all cases and confirming results obtained by univariate analysis.
Estimation of the uncertainty component due to long-term stability
The uncertainty due to long-term stability (ults) is based on the regression curve obtained for the concentration as a function of time in the long-term stability study. When the trend is not significant, the uncertainty in the determination of the angular coefficient (ua) of the regression curve can be considered as the uncertainty corresponding to the determination of the degree of degradation of the material. The uncertainty due to long-term stability (ults) can be then estimated using Eq. 1.
For this estimation, a shelf life time (t) must be defined. The longer the shelf life, the greater will be the contribution of uncertainty to stability.
Table 7 shows the angular coefficients, their associated uncertainties and the uncertainty component due to stability for a shelf life of 24 months. The angular coefficients and their uncertainties were obtained from the ANOVA tests for linear regression presented in Table 5. Also shown in Table 7 is the stability uncertainty relative to the mean concentrations obtained for each element in the flasks analyzed. The obtained values were considered acceptable for all the analyzed elements.
Conclusions
The short-term and long-term stability for the bovine kidney reference-material candidate was assessed, at a 95% confidence level, by regression analysis, analysis of variance and confirmed by multivariate analysis for As, Cl, Co, Cu, Fe, Mg, Mn, Na, Se and Zn. No trends were observed in the results with respect to the exposition period at the different chosen temperatures. In the short-term study, no statistically significant differences were observed between units exposed to the different temperatures, showing that the reference material is stable enough and may be transported under normal transportation conditions and the uncertainty component due to short-term stability can be neglected. In the long-term study, no trends were observed in the evaluated period. The uncertainty component due to long-term stability was estimated for a period of 24 months, so the future certified values and uncertainties will remain valid for this period if the reference material is stored at room temperature.
References
FAO (Food and Agriculture Organization of the United Nations) The state of food and agriculture. Food systems for better nutrition, 2013. Rome. http://www.fao.org/publications/sofa/2013/en/. Accessed 20 July 2017
FAO (Food and Agriculture Organization of the United Nations). Food balance. http://faostat3.fao.org/browse/FB/*/E. Accessed 20 March 2017
Castro L, Moreira EG, Vasconcellos MBA (2017) Use of INAA in the homogeneity evaluation of a bovine kidney candidate reference material. J Radioanal Nucl Chem 311:1291–1298
Lamberty A, Schimmel H, Pauwels J (1998) The study of the stability of reference materials by isochronous measurements. Fresenius J Anal Chem 360:359–361
Linsinger TPJ, Pauwels J, Van der Veen AMH, Schimmel H, Lamberty A (2001) Homogeneity and stability of reference materials. Accred Qual Assur 6:20–25
Van der Veen AMH, Linsinger TPJ, Lamberty A, Pauwels J (2001) Uncertainty calculations in the certification of reference materials. 3. Stability study. Accred Qual Assur 6:257–263
Pauwels J, Lamberty A, Schimmel H (1998) Quantification of the expected shelf-life of certified reference. Fresenius J Anal Chem 361:395–399
Linsinger TPJ, Van der Veen AMH, Gawlik BM, Pauwels J, Lamberty A (2004) Planning and combining of isochronous stability studies of CRMs. Accred Qual Assur 9:464–472
Jolliffe IT (2002) Principal component analysis, 2nd edn. Springer, New York
Wold S, Esbensen K, Geladi P (1987) Principal component analysis. Chemometr Intell Lab Syst 2:37–52
Lima DC, Santos AMP, Araujo RGO, Scarminio IS, Bruns RE, Ferreira SLC (2010) Principal component analysis and hierarchical cluster analysis for homogeneity evaluation during the preparation of a wheat flour laboratory reference material for inorganic analysis. Microchem J 95:222–226
Santos AMP, Santos LO, Brandao GC, Leao DJ, Bernedo AVB, Lopes RT, Lemos VA (2015) Homogeneity study of a corn flour laboratory reference material candidate for inorganic analysis. Food Chem 178:287–291
Carvalho Rocha WF, Nogueira R (2011) Use of multivariate statistical analysis to evaluate experimental results for certification of two pharmaceutical reference materials. Accred Qual Assur 16:523–528
Carvalho Rocha WF, Nogueira R, da Silva GEB, Queiroz SM, Sarmanho GF (2013) A comparison of three procedures for robust PCA of experimental results of the homogeneity test of a new sodium diclofenac candidate certified reference material. Microchem J 109:112–116
Sarembaud J, Pinto R, Rutledge DN, Feinberg M (2007) Application of the ANOVA-PCA method to stability studies of reference materials. Anal Chim Acta 603:147–154
ISO (International Organization of Standardization) (2006) Certification of reference materials. General and statistical principles. 3rd edn. ISO Guide 35. ISO, Geneva
Linsinger TPJ, Pauwels J, Lamberty A, Schimmel H, Van der Veen AMH, Siekmann L (2001) Estimating the uncertainty of stability for matrix CRMs. Fresenius J Anal Chem 370:183–188
Statsoft, Inc. (2005) STATISTICA 7.0 software, Tulsa, Oklahoma, USA
Acknowledgements
The authors would like to acknowledge the financial support of CNPq (Brazilian National Council for Scientific and Technological Development), from Research fellowship 307093/2013-1. The author L. Castro would like also to acknowledge the Ph. D. fellowship from CAPES (Coordination for the Improvement of Higher Education Personnel).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, L., Moreira, E.G., Vasconcellos, M.B.A. et al. Stability assessment of a bovine kidney reference-material candidate. J Radioanal Nucl Chem 317, 1133–1139 (2018). https://doi.org/10.1007/s10967-018-5928-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5928-8