Abstract
Sorption of micro- and microamounts of Sr from seawater has been studied using granulated Na-birnessite. Distribution coefficients of 90Sr in the natural seawater are 0.8–1.2 × 103 ml g−1, in the model seawater they are 1.6–1.8 × 103 ml g−1. Application of Na-birnessite was shown to be prospective in sorption–desorption–regeneration regime. In dynamic sorption conditions, over 150 bed volumes of seawater can be purified till 5% breakthrough occurs at feed rate 10 BV h−1. Na-birnessite can be used for 90Sr radionuclide removal from liquid radioactive wastes containing seawater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, countries as Japan, South Korea and China actively build and operate nuclear power plants (NPPs). Some NPPs situated in the coastal zone use seawater for direct cooling thus setting up possible risks. E.g., in case of an accident, huge amounts of liquid radioactive wastes (LRW) of complex chemical composition can be formed and hazardous man-made radionuclides can be brought into the ocean as happened in 2011 on Fukushima-1 NPP [1, 2]. Besides, operating and shutting down nuclear submarines and vessels equipped with nuclear power generators are under way that inevitably cause accumulation of LRW of low activity level containing seawater (SW) [3, 4].
The most hazardous man-made radionuclides are 137,134Cs and 90Sr, which possess high migration ability and easily fit into biochemical cycles. The milestone of processing low-active LRW is transferring them into industrial wastes by preliminary concentrating 137Cs and 90Sr via various methods [5, 6]. If 137Cs can be easily removed by ferrocyanide sorbents or by resorcinol–formaldehyde resins, removal of 90Sr, especially from solutions with high contents of Mg2+ and Ca2+, is still an urgent issue [7].
To remove Sr from liquid medium various sorbents are used: synthetic and natural zeolites, barium silicate and sorbents based on titanium and manganese oxides [8,9,10,11,12,13,14,15,16,17].
Although natural and synthetic zeolites are widely applied as sorption materials, their low selectivity does not allow removing 90Sr from SW [8, 9]. Only monolith Zeolite A can be an alternative to the immobilized beds of sorbent. Despite the high capacity with respect to Sr (5.1 mg-eq g−1) in dynamic conditions the breakthrough occurs after 50 bed volumes [10].
Sorption-reagent materials based on barium silicates are another group of sorbents showing high selectivity to Sr2+ ions [11] due to peculiar sorption mechanism [12, 13]. Their main drawbacks are low hydromechanical stability and irreversible sorption that limits using these materials in sorption–regeneration cycles [18].
Sorbents based on hydrated titanium oxides and titanates of alkaline metals (SrTreat sorbent) were shown to be good at removing 90Sr from alkaline solutions with high Na+ content (58 g L−1) [14, 17]. There is also known a commercial sorbent T-5 and its modification T-55, sorption properties of which were studied in the presence of Ca2+. Mean Kd value of 90Sr in the range of Ca2+ concentration 0.05–110 g L−1 is 500 ml g−1 and 320 ml g−1 for T-5 and T-55 sorbents, respectively. Sorbent based on titanosilicates of alkaline metals “IONSIV IE-911” can be used for simultaneous removal of 137Cs and 90Sr from mineralized media with high Na+ content. However, titanosilicates can hardly be implemented for 90Sr removal from SW due to low selectivity [9, 11, 19].
Manganese oxides attract much interest as sorption materials for Sr removal from complex saline media including SW. Materials based on hydrated manganese oxides and its mixtures with titanium oxides are well known. It is noteworthy that sorption on manganese oxides proceeds reversibly, therefore making possible their repeatable use in sorption–regeneration cycle. Manganese oxides are known to enhance Sr uptake with the increase of solution pH [20, 21]. In Hasany et al. [20] this is addressed to hydrolysis of Sr2+ in liquid medium according to the scheme (1):
Alkaline medium shifts the equilibrium to the right followed by sorption of Sr(OH)+ on the surface of the manganese oxide sorbent. However, such hydrolytic sorption occurs only if the sorbent possesses functional OH-group. Acidic medium suppresses hydrolysis followed by reduction of the sorption values that can be used to elute absorbed strontium [20].
Selectivity of hydrated manganese oxides increases in the row Mg2+ < Ca2+ < Sr2+ < Ba2+ that implies removing 90Sr from solutions containing even Mg2+ and Ca2+ ions. In the work [8] that studied different materials, sorbents based on hydrated and dehydrated oxides (ISMG, ISMP-1 sorbents) were the most efficient in the seawater. To date there are sorbents based on manganese oxides that were successfully used for processing liquid radioactive wastes from 90Sr. E.g., in FSUE IE “Mayak”, ISM-S and ISM-SP sorbents, which are hydrated manganese oxide sorbents, allowed processing more than 2000 bed volumes of moderately mineralized LRW [15, 16]. Specific type of hydrated Mn and Ti oxide sorbents [22, 23] appeared to be efficient in processing highly active LRW from the Savannah River Site with the total mineralization of 400 g L−1 and Na+ being the main constituent. However, Ca2+ ions suppress the Sr sorption by these materials, thus limiting implementation of these sorbents for seawater decontamination [22, 23].
The most interesting Sr sorbents are layered and tunnel manganese oxides. Birnessite is a layered manganese oxide and can be considered as an efficient sorbent for Sr removal in presence of chemically close elements. Birnessite is a precursor for producing tunnel manganese oxides [24] and can be easily obtained using available methods and equipment. In Gray et al. [25] sorption capacity of K-birnessite with respect to alkali-earth metals was shown to increase with the size contraction of hydrated cation, while Ca and Mg exchange proceeds only by 50% [26]. In Dyer et al. [27] there were demonstrated two types of sorption cites for birnessite: A cites—binding alkali and alkali-earth metals, B cites—binding Co2+, Zn2+, Cu2+ and Mn2+ ions.
Tunnel manganese oxides, todorokite and cryptomellan, can be obtained by birnessite autoclaving at 140–170 °C for several days [28,29,30]. Sorption by manganese oxides with tunnel structure is governed by ion-sieve effect, i.e. ion retention occurs if pore diameter and effect cation radius are comparable [24]. These materials have satisfactory selectivity towards Sr even in the presence of Mg2+ and Ca2+ [31, 32].
Manganese oxides are fine powders that cannot be used in dynamic sorption regime. To overcome this, manganese oxide should me mixed with binding agent or it is applied on a mechanically stable matrix, thus complicating the sorbent’s production. Possible binding agents or matrixes are SiO2 [33], methylmethacrylate [34], alginate [35], fibers of chitosan-melanin complexes [36] and polyurethane foam [37]. More than 100 m3 of LRW with activity of 89–257 Bq ml−1 was processed by composite sorbent with polyurethane foam used as a support.
Brief analysis of references has shown that most of the materials for Sr removal are low effective in the solution of complex salinity like SW. In some cases, despite high efficiency, Sr sorption can proceed irreversibly that excludes repeatable usage of the sorbents. Key-point of the modern safe management of LRW consists in minimizing volume of radioactive wastes (RW) that are sent to be fixed. That is why to implement an efficient technology for LRW processing one needs materials with high selectivity and ability to be repeatedly used in sorption–elution–regeneration cycle. Obtained radionuclide eluate then could be evaporated to a minimal volume. Fairly simple synthesis without autoclaving, well known sorption properties and satisfactory selectivity to Sr make birnessite the most preferable material to solve the mentioned technological problem.
Despite the great number of works related to birnessite sorbent, there is no information on application of this material for removing 90Sr from seawater in dynamic conditions. The work studies sorption-selective characteristics of granulated sorbent Na-birnessite and investigates the process of 90Sr removal from seawater in dynamic conditions of sorption–desorption–regeneration cycle regime.
Experimental
Materials and reagents
Potassium permanganate (KMnO4), sodium hydroxide (NaOH), hydrogen peroxide (H2O2) (25–30%), hydrochloric acid (HCl) and strontium (stable) chloride (SrCl2 × 6H2O) were purchased from Nevareaktiv, Russia. All chemicals were of analytical grade and used without additional purifications. Carrier free radio-strontium (90Sr) was obtained from Institute for Physics and Power Engineering named after A. I. Leypunsky as a chloride in 1 M hydrochloric acid solution and was diluted before use.
Seawater (SW) used in this work was collected from Amur Bay basin (Primorskiy krai, Russia) and was preliminary filtered through the Whatman “Blue Ribbon” filter with 3 μm pores. Simulated SW (S-SW) was prepared according to Kester et al. [38]. Main characteristics of the liquid media used in the work are given Table 1.
A series of model solutions with different content of Mg2+ and Ca2+ were used in the work (Table 1, solutions MS-1, MS-2, MS-3, MS-4). In the first case we prepared a series of solutions without Ca2+ and with various Mg2+ content (0.002–2.4 g L−1), in second, on the contrary, we prepared solutions without Mg2+ but with various Ca2+ content (0.004–4 g L−1). We used solutions either with stable Sr isotope with concentration 50 mg L−1 (MS-2, MS-4) or the radionuclide 90Sr with activity 1000 Bq ml−1 (MS-1, MS-3).
Synthesis of materials
Sorbent was obtained by interaction between 10 g of KMnO4 and 250 ml of concentrated H2O2. Brown precipitate was filtered under vacuum and washed with 250–500 ml of deionized H2O and then dried at 75 °C for 6 h. Obtained product was ground and held in 3 M solution of NaOH, m/V ratio 0.1–0.15 g ml−1 for 24 h. The part of material was dried at 105 °C for 6 h, the obtained sorbent is denoted as MnO2(105). After that the material was annealed at 500 °C in air for 6 h, heating rate 8.3 °C/min. Final sorbent formed granules of irregular shape, black color with characteristic metal luster and bulk weight of 1.56 g ml−1. Material was denoted as MnO2(500).
Material denoted as MnO2(500)HNa was obtained via the following procedure. Sorbent MnO2(500) (0.2 g) was put into column of 5 mm in diameter and then it was consequently fed up with 0.5 M solution of HCl and 1 M solution of NaOH at a rate of 10 bed volumes per hour (BV h−1). After that, the material was washed with 50 ml of water and dried.
Sorption of Sr in static conditions
Sorption of Sr in static conditions was carried out under continuous stirring of the sorbent and liquid media in polypropelene 10 ml cylinder at a rate of 20–30 rounds per minute on the vertical rotary shaker for 24 h, m/V—0.001 g ml−1, sample mass—0.01 g. Initial activity of the solutions was 1000 Bq ml−1. Liquid medium was separated from sorption material via decantation followed by filtration through the “Blue Ribbon” filter fixed in the Swinnex filter holder (2.5 cm in diameter). Then we measured residual activity and calculated distribution coefficient of 90Sr (Kd) or the sorption of stable isotope (S%).
Distribution coefficient of 90Sr was evaluated by formula (2):
90Sr (%) sorption was calculated by formula (3):
where A0—initial activity of the liquid medium (Bq ml−1) or the initial concentration of Sr (mg L−1), A1—residual activity of liquid medium after sorption (Bq ml−1) or equilibrium concentration of Sr (mg L−1), V—volume of liquid phase after sorption (ml), m—sorbent’s mass (g).
Sorption isotherm was obtained using S-SW series of solutions with various initial Sr2+ concentrations. Sorption time was 7 days.
Sorption of Sr in dynamic conditions
Sorbents characteristics were studied in dynamic conditions using repeated sorption–elution–regeneration cycles. Prior to the experiment 1 ml of the sample was held 1 day in the liquid medium of SW or S-SW. Then the sorbent was transferred to a glass column with inner diameter 0.5 cm through which seawater containing 90Sr (1000 Bq ml−1) radiolabel was passed at a rate of 10 columns per hour (BV h−1). To determine sorbent dynamic exchange capacity we used S-SW solution containing stable Sr of concentration 50 mg L−1 to reduce the time of the experiment. Decrease of strontium sorption efficiency up to 95% or lower we considered as a breakthrough. Retained Sr was eluted by 0.5 M HCl solution fed with 10 BV h−1 speed. After that the sorbent was regenerated with 100 ml of 1.0 M solution of NaOH, washed with 30 ml of distilled water and used in the next sorption cycle.
Sorption (%) under dynamic conditions was calculated by using the formula (3). Desorption of 90Sr was calculated by the formula (4):
where A1—eluate activity (Bq ml−1), V—eluate’s volume (ml), A x —activity of 90Sr retained during sorption (Bq), Ax−1—residual activity of 90Sr from previous cycle (Bq), x—cycle’s ordinal number, i—eluate’s fraction ordinal number.
Equipment
Content of 90Sr in seawater samples was determined on a liquid scintillation alpha–beta spectrometric radiometer Tri-Carb 2910 TR (Perkin Elmer, USA). Element’s content in the SW was evaluated using atomic absorption flame spectroscopy on a Thermo Solar AA M6 (“Thermo Electron Corporation”, USA). XRD analysis was carried out on a D8 ADVANCE diffractometer, XRD-patterns were recorded in the 2θ range 3–85° with 0.02° step and sampling time of 0.6 s per point. Phase composition was identified using “MATCH!” software and Crystallography Open Database (COD). Surface morphology was investigated by the means of scanning electron microscopy (SEM) on a device Carl Zeiss Crossbeam 1540-XB (Germany).
Results and discussion
Materials’ notation, their sorption characteristics as well as sodium and potassium content are given in Table 2.
High selectivity with respect to removed cation is one of the most important characteristics of a sorbent used for processing highly mineralized liquid media, like seawater, from 90Sr. Table 2 (columns 6, 7) presents values of Kd 90Sr obtained in SW and S-SW and indicating some trends. E.g., with the increase of annealing temperature from 105 to 500 °C efficiency of radionuclides removal increases by more than 1.5. Probably, this is caused by formation of evident crystal phase of birnessite at higher temperatures. Preliminary sequential washing of the samples with HCl and NaOH also increases the Sr uptake efficiency. Such results can be explained by the fact that treatment of manganese oxide with NaOH does not allow full substitution of K by Na. However, treatment of the final sorbent with HCl solution removes K followed by its full substitution by Na after NaOH treatment. The hypothesis is proved by chemical analysis of the samples presented in Table 2 (columns 4 and 5) showing initial manganese oxide to have high K content. Sample washed with HCl and NaOH as well as the sample after three sorption–elution–regeneration cycles contains practically no potassium. Ivanets et al. [32] showed mesoporous manganese oxide removes Sr in 0.1 M solution of NaCl better than in distilled water, thus proving Na-form birnessite to be more efficient in scavenging Sr.
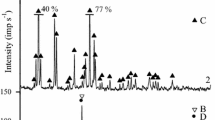
XRD patterns of the MnO2(105), MnO2(500), MnO2(500)HNa are presented on Fig. 1. Materials are characterized by low crystallinity that complicates precise phase identification. However, obtained XRD patterns allows expecting several phases, mainly K- and Na-birnessite (see legend on Fig. 1).
SEM images of manganese oxide surface are given on Fig. 2. It can be seen that original samples are monolith granules of irregular shape (Fig. 2a, b), but sequential treatment of them with HCl and NaOH changes surface morphology (Fig. 2c–e) probably improving sorption-selective characteristics. It is noteworthy that sequential purging of HCl and NaOH leads to mass loss of the material (5% in average) evidencing the sorbent’s dissolution and increasing specific surface area from 9.4 to 12 m2 g−1 (see Table 2). Despite the material’s dissolution it can be still used in dynamic conditions, because three cycles of sorption–regeneration have no effect on sorbent’s granulation (Fig. 3f–h).
Even though materials based on manganese oxides effectively retain Sr in the presence of Na+ [22], it is obvious that sorption is greatly affected by Ca2+ and Mg2+ ions that are close chemical counterparts of Sr. Table 3 presents results of Sr sorption (stable isotope and 90Sr) from model solutions containing Ca2+ and Mg2+ in various concentrations. Obviously, Ca2+ ions cause the most negative influence on Sr uptake. However, in model solution containing Ca2+ 0.4 g L−1 value Kd 90Sr are high enough indicating high selectivity of the sorption material and its applicability for radionuclides removal from seawater.
Isotherm of Sr sorption from S-SW by MnO2(500)HNa sorbent is given on Fig. 3. Fitting was carried out using classical Langmuir equation and SigmaPlot software (ver. 13.0). Isotherm is presented as a variable of removed Sr (Adsorbed Sr, mg g−1) depending on its equilibrium concentration (equilibrium, mg L−1). Evaluated saturation sorption value is 8.8 mg g−1 (Sr/sorbent). Evaluated capacity is comparable with the values for commercial ion-exchange resin Sr Resin (Eichrom Technologies Inc [39] и TrisKem International [40]). However, such values of sorption capacity can be achieved only in acidic media. Taking into account full exchange and total Sr content in SW, sorbent’s capacity before breakthrough should exceed 1000 bed volumes.
In fact, sorption of Sr from the S-SW with the particular concentration of the ion (50 mg L−1) in dynamic conditions reached 9.16, 7.82, 6.85 mg g−1 (Sr/sorbent) in the first, second and third cycles, respectively (Fig. 4a). Such capacity reduction can be caused by gradual material’s dissolution. After three cycles, the height of sorption layer reduced by 20%. Solubility can be explained by disproportionation of Mn+3 in the acidic medium into Mn+4 and Mn+2 [41].
While removing microquantities of 90Sr from real SW, sorbent’s capacity with respect to Sr content reduces and does not reach the maximal value. Capacity reduction is due to organic pollutants and organic carbon in the SW leading to reduction of Mn to lower oxidation states.
90Sr uptake from SW is peculiar due to low efficiency of radionuclides removal in the first cycle (Fig. 4, curve 1) caused by the sorbent’s turning on into the operating regime, transition into Na-form and formation of developed surface. Second and third cycles show sorbent’s capacity to exceed 150 bed volumes, while changes of the sorbent’s color or filtrate coloring were not revealed.
Elution curves of 90Sr retained from real SW in dynamic conditions are given on Fig. 5a . Although obtained results evidence instable elution of radionuclide, residual 90Sr activity in the sorbent is not higher than 4% of the total activity removed by the sorbent after 4 cycles. Treatment with NaOH is followed by insignificant desorption of residual 90Sr (Fig. 5b) reaching 0.9, 1.1, 0.7 and 0.02% from the total activity retained in the cycle.
Synthetic organic complexants like EDTA as well as oxalate salts salts are used both as cleansing agents and as a part of deactivating mixtures and washing means on nuclear power plants. That is why we studied sorption-selective characteristics of the sorbent in static conditions in the presence of Trilon B and oxalate ion (Table 4). Oxalate-ion was shown to have no effect on sorption-selective properties of the material based on manganese oxide. However, concentrations of oxalate-ions higher than 0.04 g L−1 in SW and S-SW lead to formation of insoluble precipitates of calcium and magnesium followed by co-precipitation of 90Sr.
Presence of Trilon B negatively affects sorption-selective characteristics of the sorbent, probably due to formation of stable Mn-EDTA complexes and material’s structure distortion. E.g., Trilon B was shown to have a negative influence on sorption characteristics of materials based on manganese oxides [20, 27].
Conclusions
Obtained sorption material for 90Sr removal from seawater has been obtained via interaction of KMnO4 and H2O2 followed by NaOH treatment and annealing. Final sorption material is a low crystallized layered modification of manganese oxide, namely Na–K-birnessite. HCl treatment of birnessite followed by NaOH regeneration has been shown to improve efficiency of Sr uptake by the sorbent from seawater and simulated solutions. Presence of Ca2+ ions downgrades sorption efficiency of birnessite sorbent. Nevertheless, even at the concentration of Ca 0.4 g L−1 the mean coefficient of 90Sr distribution is 1.9 × 103 ml g−1, therefore allowing the use of the material seawater treatment. Distribution coefficients of 90Sr are 0.8–1.2 × 103 ml g−1 in natural seawater, 1.6–1.8 × 103 ml g−1 in simulated seawater and the evaluated value of maximal capacity is 8.8 mg g−1. In dynamic regime, material is mechanically stable and preserves granulation. However, under long term operation, the dynamic exchange capacity of the sorbent reduces due to its dissolution. That is why long term material’s exploitation requires regular addition of new sorbent. The mean volume of seawater fed before the breakthrough of 90Sr is 150 bed volumes at the filtration rate 10 BV h−1. Presence of Trilon B makes the material less efficient due to its decomposition, which is why purified LRW should not contain organic complexing agents. The material described herein should be used for processing liquid radioactive wastes containing seawater from 90Sr radionuclide.
References
Farid O, Shih K, Lee WE, Yamana H (2013) Fukushima: the current situation and future plans. In: Lee WE, Ojovan MI, Jantzen CM (eds) Radioactive waste management and contaminated site clean-up. Woodhead Publishing, Cambridge, pp 744e–776e
FUKUSHIMA DAIICHI: ANS Committee Report. http://fukushima.ans.org/. http://fukushima.ans.org/report/Fukushima_report.pdf. Accessed 12 Mar 2018
Zheleznov VV, Vysotskii VL (2002) Application of fibrous carbon ferrocyanide sorbents for removing cesium and cobalt from large volumes of sea water. At Energy 92:493–500
Malyshev SP (1999) Small mobile installation for reprocessing of radioactive waste from technological circuits of nuclear power facilities of nuclear submarines subject to recycling. In: Sarkisov AA, du Clos AT (eds) Analysis of risks associated with nuclear submarine decommissioning, dismantling and disposal. Springer, Dordrecht, pp 405–418
Avramenko VA, Egorin AM, Papynov EK et al (2017) Processes for treatment of liquid radioactive waste containing seawater. Radiochemistry 59:407–413
Harjula R, Lehto J, Tusa EH, Paavola A (1994) Industrial scale removal of cesium with hexacyanoferrate exchanger—process development. Nucl Technol 107:272–278
Milyutin VV, Nekrasova NA, Kharitonov OV et al (2016) Sorption technologies in modern applied radiochemistry. Sorpt Chromatogr Process 16:313–322 (in Russian)
Bengtsson GB, Bortun AI, Strelko VV (1996) Strontium binding properties of inorganic adsorbents. J Radioanal Nucl Chem Art 204:75–82
Marinin DV, Brown GN (2000) Studies of sorbent/ion-exchange materials for the removal of radioactive strontium from liquid radioactive waste and high hardness groundwaters. Waste Manag 20:545–553
Sachse A, Merceille A, Barré Y et al (2012) Macroporous LTA-monoliths for in-flow removal of radioactive strontium from aqueous effluents: Application to the case of Fukushima. Microporous Mesoporous Mater 164:251–258
Brähler G, Zulauf A, Avramenko VA, Sokolnitskaya T (2014) Absorbers for removal of Sr-90 from sea water at FUKUSHIMA Site-14184. http://www.wmsym.org/. http://www.wmsym.org/archives/2014/papers/14184.pdf. Accessed 12 Mar 2018
Avramenko VA, Burkov IS, Golikov AP et al (2004) Sorption of strontium by sorptive-reagent materials. Russ J Phys Chem A 78:407–410
Sokol’nitskaya TA, Avramenko VA, Burkov IS et al (2004) Precipitation during the absorption of strontium with sorptive-reagent materials. Russ J Phys Chem A 78:411–415
Lehto J, Brodkin L, Harjula R, Tusa E (1999) Separation of radioactive strontium from alkaline nuclear waste solutions with the highly effective ion exchanger SrTreat. Nucl Technol 127:81–87
Logunov MV, Skobtsov AS, Soldatov BV et al (2004) Research and application of inorganic selective sorbents at Mayak PA. C R Chim 7:1185–1190
Voroshilov YA, Logunov MV, Prokof’ev NN, Zemlina NP (2003) ISM-S sorbent: properties and tests in a sorption process for treatment of water from accumulating basin of the Mayak production association to remove 90Sr. Radiochemistry 45:64–67
Sylvester P, Clearfield A (1998) The removal of strontium and cesium from simulated Hanford groundwater using inorganic ion exchange materials. Solv Extr Ion Exch 16:1527–1539
Avramenko VA, Zheleznov VV, Kaplun EV et al (2001) Sorption recovery of strontium from seawater. Radiochemistry 43:433–436
Milyutin VV, Nekrasova NA, Yanicheva NY et al (2017) Sorption of cesium and strontium radionuclides onto crystalline alkali metal titanosilicates. Radiochemistry 59:65–69
Hasany SM, Chaudhary MH (1981) Adsorption studies of strontium on manganese dioxide from aqueous solutions. Int J Appl Radiat Isot 32:899–904
Singh Om Vir, Tandon SN (1977) Studies on the adsorption of cesium and strontium radionuclides on hydrated manganese oxide. Int J Appl Radiat Isot 28:701–704
Kirillov SA, Lisnycha TV, Pendelyuk OI (2006) Appraisal of mixed amorphous manganese oxide/titanium oxide sorbents for the removal of strontium-90 from solutions, with special reference to Savannah river site and chernobyl radioactive waste simulants. Adsorpt Sci Technol 24:895–906
Pendelyuk OI, Lisnycha TV, Strelko VV, Kirillov SA (2005) Amorphous MnO2–TiO2 composites as sorbents for Sr2+ and UO2 2+. Adsorption 11:799–804
Feng Q, Kanoh H, Ooi K (1999) Manganese oxide porous crystals. J Mater Chem 9:319–333
Gray MJ, Malati MA (1979) Adsorption from aqueous solution by δ-manganese dioxide I. Adsorption of the alkaline-earth cations. J Chem Technol Biotechnol 29:127–134
Al-Attar L, Dyer A (2007) Ion exchange in birnessite. Land Contam Reclam 15:427–436
Dyer A, Pillinger M, Harjula R, Amin S (2000) Sorption characteristics of radionuclides on synthetic birnessite-type layered manganese oxides. J Mater Chem 10:1867–1874
Feng Q, Kanoh H, Miyai Y, Ooi K (1995) Metal ion extraction/insertion reactions with todorokite-type manganese oxide in the aqueous phase. Chem Mater 7:1722–1727
Feng Q, Yanagisawa K, Yamasaki N (1996) Transformation of manganese oxides from layered structures to tunnel structures. Chem Commun 14:1607–1608
Feng Q, Yanagisawa K, Yamasaki N (1998) Hydrothermal soft chemical process for synthesis of manganese oxides with tunnel structures. J Porous Mater 5:153–162
Dyer A, Pillinger M, Newton J et al (2000) Sorption behavior of radionuclides on crystalline synthetic tunnel manganese oxides. Chem Mater 12:3798–3804
Ivanets AI, Katsoshvili LL, Krivoshapkin PV et al (2017) Sorption of strontium ions onto mesoporous manganese oxide of OMS-2 type. Radiochemistry 59:264–271
White DA, Labayru R (1991) Synthesis of a manganese dioxide-silica hydrous composite and its properties as a sorption material for strontium. Ind Eng Chem Res 30:207–210
Valsala TP, Joseph A, Sonar NL et al (2010) Separation of strontium from low level radioactive waste solutions using hydrous manganese dioxide composite materials. J Nucl Mater 404:138–143
Hong H-J, Kim B-G, Hong J et al (2017) Enhanced Sr adsorption performance of MnO2-alginate beads in seawater and evaluation of its mechanism. Chem Eng J 319:163–169
Veleshko AN, Kulyukhin SA, Veleshko IE et al (2008) Sorption of radionuclides from solutions with composite materials based on Mikoton natural biopolymer. Radiochemistry 50:508–514
Rao SVS, Mani AGS, Karua S et al (2016) Treatment of liquid wastes using composite resins. J Radioanal Nucl Chem 307:463–469
Kester DR, Duedall IW, Connors DN, Pytkowicz RM (1967) Preparation of artificial seawater1. Limnol Oceanogr 12:176–179
Sr Resin. Eichrom Technologies Inc. https://www.eichrom.com/eichrom/products/sr-resin/. Accessed 8 Mar 2018
SR Resin product sheet. In: TrisKem International. http://www.triskem-international.com/ru/iso_album/ft_resine_sr_ru_160927.pdf. Accessed 8 Mar 2018
Drits VA, Silvester E, Gorshkov AI, Manceau A (1997) Structure of synthetic monoclinic Na-rich birnessite and hexagonal birnessite; I, results from X-ray diffraction and selected-area electron diffraction. Am Miner 82:946–961
Acknowledgements
Equipment of CUC “Far Eastern Center of structural investigations” was used in the work. The work was carried out under the financial support of Russian Science Foundation (Project No. 14-13-00135).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egorin, A., Sokolnitskaya, T., Azarova, Y. et al. Investigation of Sr uptake by birnessite-type sorbents from seawater. J Radioanal Nucl Chem 317, 243–251 (2018). https://doi.org/10.1007/s10967-018-5905-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5905-2