Abstract
In this work, the ratio contribution of 226Ra and 235U in γ spectra with the latest update nuclear data will be revised. The results showed that in the total count rate of the 186 keV peak consists of 57.2% of 226Ra (186.2 keV) and 42.8% of 235U (185.7 keV) with the existence of equilibrium. These calculations were used for determining mass activity of 238U for the direct and fast measurement using only the 186 keV peak and verifying the 238U–226Ra secular equilibrium. An equilibrium method was measured secular equilibrium of decay chain 238U (by the product daughter 214Bi-609.3 keV) that was used for this study. The maximum relative deviation was less than 4.0% between both methods proved the direct method was reliable and can be applied to determine the mass activity of 238U in the radioactive equilibrium samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural uranium consists of three isotopes 238U (99.2745%), 235U (0.72%) and 234U (0.0055%). It is conceivable that uranium within samples that originate from nuclear industry in various fields such as nuclear energy, nuclear weapons, nuclear medicine, etc. The production and using uranium have to comply with the safety radiation. The monitoring ratio activity 235U–238U was used to warn a leakage of uranium into the environment.
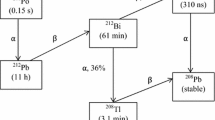
The γ spectrometry using HPGe detector has been demonstrated as a principal quantitative analysis technique for measuring of the radioactive environmental samples at many laboratories in the world. The activity of 235U is determined directly from the peak of 185.7 keV energy (γ intensity 57%). The other peaks such as 143.8 keV (10.94%), 163.4 keV (5.08%) and 205.3 keV (5.02%) are not commonly used to determine the activity of 235U because their intensity γ lower than intensity of peak 185.7 keV and the counting rates due to the peaks are often below the detection limits of the HPGe detector [1]. The activity of 238U calculates based on the activities of 214Pb (295.2, 351.9 keV), 214Bi (609.3, 1120.3, 1764.5 keV) after waiting a time deposition sample about 30 days (achieving secular equilibrium of the decay chain 238U) [2]. This method is only successful if the container sample seals off the laboratory for at least 30 days so that no radon (222Rn, the half-life 3.825 days) escape to avoid disequilibrium problems between 226Ra and 214Pb, 214Bi, its respective progenies [3]. The disadvantages of it require the prepared complex of manufacturing sample and take a long waiting time. Another method is direct using the peaks such as 63.3 keV (234Th, 3.75%) and 186.2 keV (226Ra, 3.555%). The contribution of X-ray from lead and the self-absorption of a sample would influence the result activity of 238U at the peak 63.3 keV (234Th) [4, 5]. Therefore, the peak 186.2 keV (226Ra) was chosen to calculate activity of 238U using the direct method.
When the environmental samples reached a secular equilibrium of the decay chain 238U, the activity of 226Ra is considered the equal activity of 238U. These samples had a containing both 226Ra (186.2 keV, 3.555%) and 235U (185.7 keV, 57%) of count rate at the region 186 keV. Ebaid et al. determined the ratio of contribution 226Ra and 235U with correction factors of 58.3 and 41.7%, respectively [6]. Gilmore evaluated this ratio include 57.09% (226Ra) and 42.91% (235U) at the region 186 keV [7]. Hung et al. used the RGU standard with correction factors of 57.09% to correct the 226Ra value and built the standard efficiency curve for HPGe detector in the energy range from 46.5 to 2204.2 keV [8]. In this work, we calculated the ratio contribution of 226Ra and 235U at the region 186 keV with the latest nuclear data. These results are used to perform the standard efficiency curve for HPGe detector and determined mass activity of 235U and 238U in geological samples using the direct method. In addition, the equilibrium method of the decay chain 238U was also applied to determine mass activity of 235U (185.7 keV) and mass activity of 238U calculated through the daughter such as 214Pb (351.9 keV) and 214Bi (609.3, 1764.5 keV). The relative deviation was less than 4.0% for both methods.
Materials and methods
Theoretical work
In the γ ray spectrum, the total counts of 186 keV peak will include counts of 226Ra (186.2 keV, 3.555%) and 235U (185.7 keV, 57%). We have:
The formula calculates radioactivity:
where A (Bq kg−1) is a mass activity, m (kg) is dry mass, I γ and ɛ are intensity γ ray and efficiency detector in energy E (keV), t (s) is an acquisition time, respectively.
We assume the analysis sample does not get rich and not exhaust uranium (in a state radioactive equilibrium). The dry mass sample will include 0.72% mass of 235U and 99.2745% mass of 238U [7]. If the sampling area had not changed geochemical, we considered the activity of 226Ra equal the activity of 238U (the half-life of 226Ra is 1600 years, very small compared with the half-life of 238U), the ratio count rate is:
Due to the efficiency of HPGe detector at the 186.2 keV peak equal with the efficiency at the peak 185.7 keV \((\varepsilon_{{{}^{235}{\text{U}}}}^{185.7} = \varepsilon_{{^{226} {\text{Ra}}}}^{186.2} ),\) γ intensity of 226Ra (186.2 keV) and 235U (185.7 keV) are \(I_{{^{226} {\text{Ra}}}}^{186.2} = (3.555 \pm 0.019)\%\) and \(I_{{{}^{235}{\text{U}}}}^{185.7} = (57.0 \pm 0.3)\% ,\) respectively, the half-life \(T_{{^{235} {\text{U}}}} = 7.04 \times 10^{8}\) years, \(T_{{^{238} {\text{U}}}} = 4.468 \times 10^{9}\) years [9]. The ratio of the contribution for 226Ra (186.2 keV) and 235U (185.7 keV) show that in the region 186 keV consists of 57.2% 226Ra and 42.8% 235U using Eqs. (4) and (5), respectively.
Direct method
When the samples reached a secular equilibrium of the decay chain 238U and a state radioactive equilibrium, we can calculate the mass activity (Bq kg−1) of 235U and mass activity of 238U using Eqs. (6) and (7), respectively:
where N total is counts of measured sample minus background, the relative uncertainties of mass activity were calculated following the propagation of uncertainty [10].
Equilibrium method
The samples were sieved and filled into the cylindrical container and then sealed off the laboratory at least 30 days so that no radon escape to avoid disequilibrium problems between 226Ra and its respective progenies (214Pb and 214Bi). We have:
The mass activity of 214Bi (609.3 keV, 45.49%):
From Eqs. (1), (2) and (8), the mass activity 235U (Bq kg−1) calculated to follow Eq. (10) [11], the relative uncertainties of mass activity were calculated to the law of propagation of uncertainty [10]:
The decay chain 238U includes 14 series of radioactive isotopes other and the mean of mass activity can be calculated by Eq. (11), where n for number isotopes, A i and u i is mass activity and direct uncertainty of ith isotopes, respectively.
The direct uncertainty of mean mass activity:
Experimental data
The experimental measurement was performed with a coaxial p-type HPGe, supplied by Canberra, Inc., USA, crystal diameter 62.2 mm, crystal length 50.1 mm, the relative efficiency 35% and the energy resolution (FWHM) at 1332 keV (60Co) is 2.0 keV, which is using the Lynx® based on advanced digital signal processing techniques, the spectra were recorded using Genie-2K software with 32,748 channels. The detectors are surrounded by a cylindrical low-background passive shielding made of 100 mm thickness of lead, and inside had copper and tin with thickness 1.6 and 1.0 mm, respectively. The samples are closed in a cylindrical container with external diameter 75 mm, filled to height 20 mm, the thickness of wall 2 mm and have a dry mass such as Table 1.
The efficiency calibration is experimentally determined by RGU standard (mass activity 4940 ± 30 Bq kg−1) at encap of the detector, which is used to perform the efficiency calibration curve for HPGe detector in the energy range from 46.5 to 2447.9 keV. The acquisition time is 86,400 s for background and samples. The peaks and the overlapping peaks are processed using Colegram software [12]. The samples sealed off the laboratory for at least 30 days so that no radon (222Rn, the half-life 3.825 days) escape to avoid disequilibrium problems between 226Ra and its respective progenies (214Pb and 214Bi).
The full energy peak efficiency was determined at the 14 values of 210Pb (46.5 keV), 234Th (63.3 keV), 226Ra (186.2 keV), 214Pb and 214Bi (in equilibrium with its parent 238U). We used to correction factor 0.572 to correct count of 226Ra at the region 186 keV. The experimental efficiency curve were fitted by the ACORES software [11]. The relative deviation between the values from experimental efficiency values and efficiency calibration curve was less than 2.5% (see Table 2).
Results and discussion
The mass activity of 238U (Bq kg−1) was calculated based on the activities 234Th (63.3 keV), 214Pb (351.9 keV) and 214Bi (609.3, 1764.5 keV). The maximum relative deviation between the mass activity of 238U at 63.3 keV (234Th) and the mean of mass activity is less than 6.0%. Equation (7) was used for determining mass activity of 226Ra (186.2 keV). The maximum relative bias between the mass activity of 238U calculated by the direct method using the 186.2 keV peak (226Ra) and the mean of mass activity of 238U is less than 3.0% (see Table 3).
That shows the samples M1–M4 achieved secular equilibrium between 238U and its respective progenies (214Pb, 214Bi). The radioactivity of the 226Ra calculated for the samples ranging from 36.49 ± 0.94 (Bq kg−1) to 277.00 ± 3.67 (Bq kg−1). It is mass activities of the sample in this study are higher than the activity concentration of 226Ra for rock samples [13], and soil sample [14] in the Ramanagara and Tumkur districts, Karnataka, India. Figure 1 shows to compare between background and M1 sample.
In the other hand, the direct method [Eq. (6)] and the equilibrium method [Eq. (10)] are used to determine the mass activity of 235U for the samples. Table 4 presents a good agreement between both methods was less than 4.0%.
Conclusions
The revision of nuclear data shows that the 186 keV peak has contributed count of 226Ra and 235U about 57.2 and 42.8%, respectively. A direct analysis method with correction factor 0.572 for 226Ra value and 0.428 for the 235U value can be determined mass activity of 226Ra and 235U in the samples with the existence of equilibrium. In this paper, the radioactivities of the 238U and 235U can be calculated, which is a good agreement with both methods. Moreover, the advantage of the direct method can be applied after preparation sample, which does not need for the secular equilibrium between 226Ra and its progenies (214Pb and 214Bi).
References
Corte FD, Umans H, Vandenberghe D, Wispelaere AD, Van den Haute P (2005) Direct gamma-spectrometric measurement of the 226Ra 186.2 keV line for detecting 238U/226Ra disequilibrium in determining the environmental dose rate for the luminescence dating of sediments. Appl Radiat Isot 63:589–598
Ebaid YY (2010) Use of gamma-ray spectrometry for uranium isotopic analysis in environmental samples. Rom J Phys 55:69–74
Völgyesi P, Kis Z, Szabó Z, Szabo C (2014) Using the 186-keV peak for 226Ra activity concentration determination in Hungarian coal-slag samples by gamma-ray spectroscopy. J Radioanal Nucl Chem 302:375–383
Huy NQ, Luyen TV (2004) A method to determine 238U activity in environmental soil samples by using 63.3-keV-photopeak-gamma HPGe spectrometer. Appl Radiat Isot 61:1419–1424
Thanh TT, Loan TTH, Nhon MV, Tao CV (2014) Improvement of passive shielding to reduce background components to determinate radioactivity at low energy gamma rays. Kerntechnik 79:247–252
Ebaid YY, El-Mongy SA, Allam KA (2005) 235U-γ emission contribution to the 186 keV energy transition of 226Ra in environmental samples activity calculations. Int Congr Ser 1276:409–411
Gilmore G (2008) Practical gamma-ray spectrometry, 2nd edn. Wiley, Weinheim
Hung NQ, Chuong HD, Vuong LQ, Thanh TT, Tao CV (2016) Intercomparison NaI(Tl) and HPGe spectrometry to studies of natural radioactivity on geological samples. J Environ Radioact 164:197–201
http://www.nucleide.org/Laraweb/. Accessed 28 July 2017
GUM (2008) Evaluation of measurement data—introduction to the “Guide to the expression of uncertainty in measurement” and related document. BIPM. http://www.bipm.org
Lépy MC, Ferreux L, Hamon C, Plagnard J (2008) Logicised d’ajustement des courbes de rendement ACORES. Note tech. LNHB 2008-2045
Lépy MC (2004) Presentation of the COLEGRAM software. Note tech. LNHB 04/26
Rangaswamy DR, Srilatha MC, Ningappa C, Srinivasa E, Sannappa J (2016) Measurement of natural radioactivity and radiation hazards assessment in rock samples of Ramanagara and Tumkur Districts, Karnataka, India. Environ Earth Sci 75:373 (1–11)
Srilatha MC, Rangaswamy DR, Sannappa J (2015) Measurement of natural radioactivity and radiation hazard assessment in the soil samples of Ramanagara and Tumkur districts, Karnataka, India. J Radioanal Nucl Chem 303:993–1003
Acknowledgements
This research is funded by Vietnam National University Ho Chi Minh City under Grant Number B2017-18-02.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vuong, L.Q., Chuong, H.D., Nguyen, V.H. et al. Revision of nuclear data of 235U and 226Ra for the 186-keV gamma-ray peak for the determination of activity in environmental samples. J Radioanal Nucl Chem 314, 1273–1277 (2017). https://doi.org/10.1007/s10967-017-5461-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5461-1