Abstract
An attempt was made to understand the sorption behaviour of Pu4+ and PuO2 2+ on Rhizopus arrhizus fungus. The sorption data were analyzed using Langmuir, Dubinin–Radushkevich, Freundlich isotherm and Tempkin isotherms, which revealed that the sorption proceeds via chemisorption through mono layer following Langmuir isotherm. the sorption kinetics were analyzed by different models revealing the predominance of pseudo 2nd order kinetics. Oxalic acid and sodium carbonate were used for effective stripping of Pu4+ and PuO2 2+, respectively. This biomass was found to be radiolytically stable and finally was applied for processing of SHLW from RR and FBR origins.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A transuranic radioactive element proven being a promising factor for the success of nuclear programme, Pu as from its fissile nature it describes that it can sustain a nuclear chain reaction which is one the character that qualify Pu as fuel in reactors, it can have other applications too as used as a heat source in radioisotope thermoelectric generator in aircrafts [1, 2]. The fact that Pu is a man-made element and not found in nature, makes it expensive and as the three stages of nuclear programme in India mainly revolves around the 239Pu in first two most important stages making it more vulnerable, hence judicious and efficient use of the element is one of the prime requisite apart from the environmental risk associated with its radio-toxicity. Therefore preferential separation of plutonium ion is important in front and back end of nuclear fuel cycle. TBP is used extensively for the extraction of plutonium in nuclear industries from spent fuel [3–7]. But due its poor radiolytic stability which resulted in poor efficiency and degraded its selectivity, non-biodegradability, co-extraction of Ru, Rh, generation of large amount of organic liquid waste leads to a need of efficient, selective, radiolytically stable and environmental friendly separation procedure for Pu. Solid phase separation using biomass not only reduces the handling of large amount of organic waste, but also makes the overall separation process cheaper. Many studies have been made and divulge the fact that microbial biomass are very efficient in absorbing metal ions from the aqueous solution [8–14] as they have many potential sites as cell wall, cell envelope, etc. for the uptake of metal ions [8, 15, 16]. It was studied that these microbial biomass could be used to decontaminate waste streams of mining, nuclear fuel cycle, refinery etc., and also could be used to concentrate metals [13, 14, 17, 18]. Biomasses from a bacterial species-Streptomyces viridochromogenes, the brewer’s yeast-Saccharomyces cerevisiae, bacterialspecies-Pseudomonas aeruginosa and Rhizopus arrhizus a fungus and many others were studied for uptake of uranyl ion [13, 14, 17, 18]. R. arrhizus biomass was the most efficient one of them [14], therefore this biomass in particular draw attention of the researchers. Literatures revealed that R. arrhizus secrete small siderophores which are mainly chelating compounds of iron. These sidophores are strong iron chelating agents as they form stable hexadentate, octahedral complex preferentially with Fe3+ rather with other metal ions. It has been proven that it is also a good chelating agent for plutonium, uranium, aluminium, galium, chromium, copper, zinc and magnesium [19, 20]. In view of this, the present investigation was based on understanding the sorption behavior of plutonium in tetra and hexa valent oxidation states on R. arrhizus. It includes analyzing different sorption isotherms, sorption kinetics, stripping behavior, selectivity and radiolytic stability.
Experimental
Preparation of biomass
The fungus of Mucoraceae family, R. arrhizus (wild type) was procured from National chemical laboratory, Pune, India. Then it was cultured in a 150 ml sterilized modified Czepak box [21–23] within a 500 ml erlenmeyer flask at 28 ± 1 °C on a rotary shaker at 150 rpm. Then the biomass acquired from the Rhizopus species was washed with boiling acetone and dried at 110 °C to constant weight. Finally, the dried biomass was ground sieved and the 30–50 mesh size fraction (British standard) was used for the present investigation.
Materials
239Pu stock solution was prepared by dissolving spectra pure PuO2 in conc. HNO3 +0.05 M HF followed by fluoride removal by repeated evaporation to dryness. Finally, the aqueous feed acidity was adjusted to 1 M HNO3. The oxidation state of plutonium was adjusted using NaNO2 and AgO for Pu4+ and PuO2 2+, respectively. Oxalic acid and Na2CO3 used in the present investigation were produced from Thomas Baker Chemical limited and Qualigens fine Chemicals, Mumbai, India respectively. Suprapure HNO3 (E-Merck, Darmstadt, Germany), CertiPUR® solutions of individual elements (E-Merck, Darmstadt, Germany) and quartz double distilled water were used throughout the investigation. All the experiments were carried out with tracer level of 239Pu (µM range).
Method
Determination of K d at various feed acidity
For the sorption experiment 5 mg of biomass R. arrhizus NCIM 997 each, was taken in different tubes and allowed to equilibrated with aqueous phase containing 239Pu (µM level) in various feed acidity (ranging from 0.01–6 M HNO3) in a temperature controlled water bath shaker for 2 h. After 2 h of equilibration, it was allowed to settle for 5 min. Then suitable aliquots from the aqueous phase was collected and used for liquid scintillation counting. The K d values were calculated using the equation below [23].
where C 0 is the initial plutonium count, C e is the plutonium count after bio-sorption, v is the volume of the aqueous phase (in mL) and w is weight of the biomass (in gm) taken.
Understanding the sorption isotherm
For understanding the sorption isotherm different amount of biomass (5–25 mg) were taken for the investigation. The bio-sorption experiments were done at 300 K for 2 h.
Kinetics of sorption
For sorption kinetics experiments 20 mg biomass was allowed to equilibrate for different contact time (ranging from 15 to 180 min) at 300 K from an aqueous feed acidity 3 M HNO3.
The stripping studies
The stripping of metal ion from R. arrhizus is one of the essential requirements for the reusability of the sorbent. The stripping experiments are carried out in as a two step process. In the first step the biomass was allowed to equilibrate with the plutonium in 3 M aqueous feed acidity. In the second step, the loaded biomass was allowed to contact with 1 ml of stripping solution (1 M Na2CO3, 1 M oxalic acid). After second step the aqueous phase was collected to evaluate the % stripping behavior of Pu from loaded biomass.
Processing of simulated high level waste (SHLW)
For checking the selectivity of the R. arrhizus biomass towards plutonium ions over other metal ions; biomass was applied to process SHLW of research reactor (RR) and Fast Breeder Reactor (FBR) origin. The raffinate was then directly fed to the plasma for the analysis of the constituents metal ions by Inductive Coupled Plasma Atomic Emission Spectrometer (ICP-AES) with charged coupled device (CCD) as detector. The optimized experimental and instrumental parameters are summarized elsewhere [24]. This is a relative and simultaneously multi elemental method. CertiPUR® solutions of individual elements (E-Merck, Darmstadt, Germany) were used for multi-point calibration (0.05–1000 mg L−1). Though different analytical lines of the analytes were used, the results corresponds to the best performed analytical lines were summarized. Since only µM level of Pu was spiked in SHLW, the spectral interference from Pu was not significant, therefore, Pu separation prior to the ICP-AES analysis was not necessary. The SHLW was prepared in aqueous medium and the individual analyte concentrations were in the range of 100 of mg L−1. It has been demonstrated that these concentrations of concomitant analytes did not show any mutual interference in the specified analytical lines. Therefore, aqueous Merck standards were used for quantification.
The radiolytic stability
For the investigation on the radiolytic stability of the biomass, the biomass was exposed in gamma rays having dose up to 1000 kGy using 60Co source in FTD, Bhabha Atomic Research Centre, Mumbai, India. The K d values for Pu in both the oxidation states were determined using the irradiated biomass following same experimental procedure.
Results and discussions
Variation of K d values as a function of aqueous feed acidity
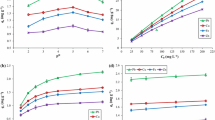
Extraction profile for Pu+4 and PuO +22 was obtained in Fig. 1 by varying K d values as a function of aqueous feed acidity. Throughout the feed acidity range the K d values for Pu4+ were found to be almost 1.5 times more than that of PuO2 2+. The higher sorption efficiency of tetra valent plutonium is attributed to the higher chemical potential compared to hexavalent plutonium [25]. For both the oxidation states the K d values were found to decrease with increase in aqueous feed acidity. This can be attributed to the fact that at higher feed acidity, the availability of large amount of H+ ion competes strongly with the plutonium ion or in other words the ligating sites in the biomass were largely utilized in the protonation [26].
Sorption isotherm
For understanding the sorption mechanism, different sorption isotherm models were taken into consideration. The experimentally obtained data of sorption for Pu+4 and PuO +22 were used for fitting in widely four famous sorption isotherm models, Langmuir, Dubinin–Radushkevich (D–R), Freundlich and Temkin isotherms and based on the best linear regression, the operating isotherm model was determined.
Langmuir isotherm
This is most accepted model which assumes the ideal nature of sorbent–sorbate interaction at isothermal condition and presumes certain facts which include that all sites of sorption are equivalent, all the absorbing sites show homogenous nature, sorption is restricted only up to monolayer and there is no interaction between adsorbate molecules on neighboring sites. This isotherm can be explained by the given equation below [27–29].
where, q e is the amount of metal ion adsorbed on the fungus biomass at equilibrium condition, C e is the equilibrium concentration of the Pu4+ or PuO2 2+ ion, q o is the sorption capacity of biomass for Pu4+ or PuO2 2+ ion in which the sorption capacity of fungus biomass for Pu4+ was higher than that of PuO2 2+ as observed from the experimental data and b is the sorption energy which is also higher for Pu4+ than PuO2 2+ as observed in the present study. The linear regression coefficient of Pu4+ and PuO2 2+ for the Langmuir isotherm were found to be 0.99998 and 0.99982 respectively.
Dubinin–Radushkevich (D–R) isotherm
Dubinin–Radushkevich isotherm model is based on the Polyani potential theory of adsorption. Dubinin–Radushkevich isotherm model can be shown as [30].
where, X m is the maximum sorption capacity, ε is the polyani potential, β is the activity constant and q e is the amount of adsorbed Pu ion at equilibrium on the R. arrhizus biomass. The, ε value could be found out from the given equation below
in which, R is the universal gas constant i.e. 8.314 J mol−1 K−1, C e is the Pu ion in equilibrium concentration and T is the absolute temperature. The E energy value can be calculated from the given equation below
The E value of Pu4+ and PuO2 2+ were found to be 537.63 and 719.42 kJ mol−1 respectively which suggests that the sorption process is chemi-sorption. The linear regression coefficient of Pu4+ and PuO2 2+ for the D–R isotherm were found to be 0.97806 and 0.97004 respectively.This high sorption energy for both the metal ion revealed that the sorption process must be through the chemical interaction and it is definitely not physic-sorption.
Temkin isotherm
The main assumption of Temkin isotherm model is that, the heat of adsorption (function of temperature) decreases linearly (not logarithmically) with increasing coverage. This isotherm also neglects the extremely low and high value of concentrations. The model can be quantitatively expressed as [31].
where, A T is Temkin isotherm equilibrium binding constant (L g−1), b is Temkin isotherm constant, and T is the absolute temperature. In our present investigation it was observed that the b value for PuO2 2+ was lower than that of Pu4+ whereas overturning result was noted for the A T values. The linear regression coefficient of Pu4+ and PuO2 2+ for the Temkin isotherm were found to be 0.99803 and 0.99823, respectively.
Freundlich isotherm
This adsorption isotherm is an experimental relation of the concentration of a solute on the surface of an adsorbent with the concentration of the solute in the liquid. This adsorption model proceeds by assuming certain facts that (i) the adsorbing sites are heterogeneous in nature (ii) adsorption is not restricted only up to monolayer (iii) also the active sites are non-uniform in nature. The following Freundlich isotherm holds good at low pressure and can be showed as [32].
where, x/m is the amount of plutonium ions adsorbed per gram of the R. arrhizus biomass at equilibrium condition (mg g−1), K f is Freundlich isotherm constant (mg g−1), C e is the equilibrium concentration of plutonium (mg L−1), n is sorption intensity and 1/n is a function of the strength of adsorption. If ‘n’ is unity, then the partition of metal ions between the solid phase and liquid phase are independent of the concentration of metal ion. ‘n’ value above unity reveals normal sorption and ‘n’ value less than one shows that the sorption process is cooperative [33]. The bio-sorption of Pu4+ and PuO2 2+ on fungus biomass reveals that the sorption process is cooperative since ‘n’ value for Pu4+ and PuO2 2+ were found below unity i.e., 0.9738 and 0.9315 respectively. The K f value for Pu4+ and PuO2 2+ from the observationshows that Pu4+ sorption capacity is more than that of PuO2 2+.The linear regression coefficient of Pu4+ and PuO2 2+ for the Freundlich isotherm were found to be 0.99950 and 0.99988 respectively.
Figure 2 represents the linear variations for Langmuir, D–R, Freundlich and Temkin isotherm analyses while Table 1 summarizes different constants and linear regression coefficients for the same.
Sorption kinetics
The kinetics of sorption is one of the important aspects to understand the sorption process. In our present study kinetics data were fitted to three widely accepted models. F, the fractional attainment of equilibrium can be shown in the equation given below.
in which C t is the amount of metal sorped on the fungus biomass at time ‘t’ and C te is the amount of metal ion sorped on fungus biomass at equilibrium.
Figure 3 shows the plot for (1−F) as a function of equilibration time which shows no appreciable variation in (1−F) for Pu4+ and PuO2 2+ ions after 120 min that reveals the fact that for attainment of equilibrium 120 min is sufficient. To get into the more insight of the sorption different models are applied as follows.
Lagergren first order rate kinetics
Lagergren pseudo-first order rate equation has been used to describe the kinetic process of liquid–solid phase adsorption in our present study, which can be shown by [34].
In which, q e is is the amount of metal ion adsorbed on fungus biomass at equilibrium condition, q is the amount of metal ion adsorbed on fungus biomass at time ‘t’ and k ads is the rate constant which can be calculated from the plot of log (q e−q) versus ‘t’ (Fig. 3). From the observation of our study it was revealed that the k ads value for PuO2 2+ was found higher than that of Pu4+. The linear regression coefficients of both Pu4+ and PuO2 2+ for Lagergren model were 0.97030 and 0.98483 respectively. The poor linear regression coefficients also revealed that probably Lagergren first order rate kinetics is not operative for the present case.
Intra particle diffusion model
Intra particular diffusion model is one of the widely accepted models in analyzing sorption kinetics [30, 35, 36] can be expressed by the given equation below [35, 36].
in which k p stands for the intra-particle diffusion rate constant and C is the intra-particle diffusion constant. From the above equation a plot for q, amount of sorbate adsorbed as a function of the square root of the time can be drawn (Fig. 3), where the slope (k p),is the rate constant and c, intercept of the plot and is directly proportional to the boundary layer thickness. Both the intra-particle diffusion rate constant and the intra-particle diffusion constant values for Pu4+ is higher than that of PuO2 2+. The linear regression coefficients of both Pu4+ and PuO2 2+ for Intra particle diffusion model were 0.85716 and 0.8817, respectively. The linear regression coefficients for the present model were found even poorer than the former model suggesting the actual sorption kinetics is far from the intra particle diffusion model.
Pseudo-second-order kinetics
Pseudo second order kinetics is expressed in the equation given below as [37].
where K 2 is the pseudo second order rate constant (unit-g mg−1 min−1). The plots of t/q as the function of t, gave the straight lines (Fig. 3). Form the slope value qe was evaluated and substitute to the intercept value to obtain K 2. The linear regression coefficients of both Pu4+ and PuO2 2+ for pseudo-second-order kinetics model were 0.9999 and 0.9997 respectively. The closeness of regression coefficients towards unity revealed the sorption proceeding via pseudo-second-order kinetics (Table 2).
As retrieving the adsorbed metal ions from the biomass is essential aspect for understanding its reusability. A series of stripping agents have been employed for the effective back extraction of Pu4+ and PuO2 2+ from the loaded biomass but only 1 M sodium carbonate or 1 M oxalic acid were found to be effective. Figure 4 showed the % stripping behavior of plutonium in different oxidation states from the biomass. It was observed that 1 M oxalic acid can strip 90 and 78% of tetra and hexa valent plutonium, respectively while the % stripping using sodium carbonate was found to be 84 and 85% respectively. This study revealed that for tetra valent plutonium oxalic acid is successful whereas for hexa valent plutonium sodium carbonate serves the purpose.
Radiolytic stability of Rhizopus arrhizus biomass
Radiation stability of biomass is an important factor for using it for extraction of metal ions from the radioactive waste as it would be going through high amount radiation and there is risk of being degraded to and forming degradation products. The sorbent to be used for the processing of radio-toxic metal ions must have very high radiolytic stability. In view of these the biomass was allowed to exposed up to gamma dose of 1000 kGy and with the irradiated biomass the K d values for tetra and hexavalent plutonium were evaluated. It was observed that after 500 kGy gamma exposure the K d value for Pu4+ became ~94% of its original value while that for PuO2 2+ becomes ~95%. After a gamma exposure of 1000 kGy the K d values for Pu4+ becomes ~86% and for PuO2 2+ it becomes ~78% (Fig. 5). The overall study revealed that this biomass is radiolytically stable to process radioactive waste solution.
Processing of the simulated high level waste solutions originating from research reactor and fast breeder reactor origin using Rhizopus arrhizus biomass
The development of the new biomaas material will be successful when it can selectively separate the targetted metal ions from complex waste matrix. In view of this the selectivity of R. arrhizus was investigated by processing SHLW solution originating from Research reactor and Fast breeder reactor origin. After processing the raffinate was fed directly to plasma for the analysis by ICP-AES. The analytical results were summarized in Table 3. The study revealed that Cr, Fe, Mn, Ni, Sr, Ce, La and Pr were co-extracted along with plutonium while the other metal ions are either not extracted or only partially extracted. Pu (in µM range) was spiked into the SHLW solutions and they were processed by the sorbent. The K d values for pure Pu solution (tracer level) was determined without adjusting the oxidation states and compared that in SHLW conditions.
In SHLW conditions, the K d values for Pu were found to decrease moderately due to the co-extraction of other metal ion on the sorbent. This will lead to decrease in the availability of the coordinating sites on sorbent.
A comparative study on the efficacy of different biomass in metal ion sorption
In the long stretch of time different biomass like Psedomonas, Chitosan, Arca shell, some fungus, some algae are being tested as sorption materials for radio nuclides in the front and back end of the nuclear fuel cycle [38]. Table 4 summarized a comparison of total evaluation of the bio mass presently under investigation with that reported in the literatures. Dhami et al. reported the high efficiency of different biomass originating from Rhizopus family only at 0.01 M HNO3 feed solution [39]. Though the extraction efficiency was found to be encouraging, the feed acidity is far from HLW solution (3 M HNO3). Moreover the literature only reports the K d values without throwing any light on the sorption mechanism, kinetics, stripping, radiolytic stability and selectivity. Similarly, Dutta et al. reported the sorption of UO2 2+, Th4+, Cs+, Eu3+, Am3+, Sr2+ ions on cross linked chitosan biomass from pH 6 [40, 41 ]. But in this investigation the reported K d values were not that high to be considered as a good sorption. Additionally, this biomass has not been evaluated for different aspects of sorption as specified earlier. In our present investigation not only the higher K d values for Pu4+ and PuO2 2+ were reported even from 3 M HNO3 feed acidity but also a total evaluation of the material was carried out including efficiency, mechanism, selectivity, stability against radiation, kinetics and stripping behavior.
Conclusions
In the present investigation, R. arrhizus was demonstrated as highly efficient and selective bio-sorption material for tetra and hexavalent plutonium ion. The sorption capacities for Pu4+ and PuO2 2+ were found to be 89.87 and 74.37 mg g−1, respectively. The very high sorption energy obtained from Dubinin–Radushkevich (D–R) isotherm (for Pu4+and PuO2 2+ 537.63 and 719.42 kJ mol−1, respectively) revealed the sorption process is chemi-sorption. The analysis of the sorption kinetics revealed that it followed pseudo second order kinetics. This R. arrhizus biomass was found to be radiolytically stable with ~20% reduction of K d values for Pu4+ and PuO2 2+ on 1000 kGy gamma exposure. Oxalic acid was found to be efficient of desorption of Pu4+ while sodium carbonate was useful for PuO2 2+. During processing of SHLW from RR and FBR origin, the biomass was found to co-extract Cr, Fe, Mn, Ni, Sr, Ce, La and Pr along with plutonium.
References
Weber WJ, Ewing RC (2000) Plutonium immobilization and radiation effects. Science 289(5487):2051–2052
Krahenbuhl MP, Slaughter DM, Wilde JL, Bess JD, Miller SC, Khokhryakov VF, Suslova KG, Vostrotin VV, Romanov SA, Menshikh ZS, Kudryavtseva TI (2002) The historical and current application of the FIB-1 model to assess organ dose from plutonium intakes in mayak workers. Health Phys 82(4):445–454
Lin Y, Wai CM, Jean FM, Brauer RD (1994) Supercritical fluid extraction of thorium and uranium ions from solid and liquid materials with fluorinated.beta-diketones and tributyl phosphate. Environ Sci Technol 28(6):1190–1193
Sato T (1965) Extraction of uranium (VI) and thorium from nitric acid solutions by tri-n-butyl phosphate. J Appl Chem 15(11):489–495
Siddall TH (1959) Trialkyl phosphates and dialkyl alkylphosphonates in uranium and thorium extraction. Ind Eng Chem 51(1):41–44
Gupta B, Malik P, Deep A (2002) Extraction of uranium, thorium and lanthanides using Cyanex-923: their separations and recovery from monazite. J Radioanal Nucl Chem 251(3):451–456
Suresh A, Srinivasan TG, Rao PRV (1994) Extraction of U(VI), Pu(IV) and Th(IV) by some tri-alkyl phosphates. Solvent Extr Ion Exch 12(4):727–744
Beveridge TJ, Koval SF (1981) Binding of metals to cell envelopes of Escherichia coli K-12. Appl Environ Microbiol 42(2):325–335
Beveridge TJ, Murray RGE (1976) Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol 127(3):1502–1518
Galun M, Keller P, Malki D, Feldstein H, Galun E, Siegel SM, Siegel BZ (1982) Removal of uranium (VI) from solution by fungal biomass and fungal wall-related biopolymers. Science 219(4582):285–286
Nakajima A, Horikoshi T, Sakaguchi T (1981) Studies on the accumulation of heavy metal elements in biological systems. XVII. Selective accumulation of heavy metal ions by Chlorella regularis. Eur J Appl Microbiol Biotechnol 12(2):76–83
Strandberg GW, Shumate SE II, Parrott JR Jr (1981) Microbial cells as biosorbents for heavy metals: accumulation of uranium by Saccharomyces cerevisiae and Pseudomonas aeruginosa. Appl Environ Microbiol 41(1):237–245
Tsezos M, Keller DM (1983) Adsorption of 226radium by biological origin absorbents. Biotechnol Bioeng 25(1):201–215
Tsezos M, Volesky B (1981) Biosorption of uranium and thorium. Biotechnol Bioeng 23:583–604
Garcia SB, Nickerson WJ (1962) Isolation, composition and structure of cell walls of filamentous and yeast like forms of Mucor rouxii. Biochim Biophys Acta 58:102–119
Crist RH, Oberhauser K, Shank N, Ngkuyen M (1981) Nature of bonding between metallic ions and algae cell walls. Environ Sci Technol 15(10):1212–1217
Horikoshi T, Nakajima A, Sakaguchi T (1981) Studies of the accumulation of heavy metal elements in biological systems. XIX. Accumulation of uranium by microorganisms. Eur J Appl Microbiol Biotechnol 12(2):90–96
Weidemann DP, Tanner RD (1981) Modelling the rate of transfer of uranyl ions onto microbial cells. Enzyme Microb Technol 3:33–40
Seth GJ, Ruggiero CE, Hersman LE, Chang-Shung Tung, Neu MP (2001) Siderophore mediated plutonium accumulation by microbacterium flavescens (JG-9). Environ Sci Technol 35(14):2942–2948
Hider RC, Hall AD (1991) Clinically useful chelators of tripositive elements. Prog Med Chem 28:41–137
Dhami PS, Gopalakrishnan V, Kannan R, Ramanujam A, Salvi NA, Udupa SR (1998) Biosorption of radionucleides by Rhizopus arrhizus. Biotechnol Lett 20(3):225–228
Dhami PS, Kannan R, Gopalakrishnan V, Ramanujam A, Salvi NA, Udupa SR (1998) Sorption of plutonium, americium and fission products from reprocessing effluents using Rhizopus arrhizus. Biotechnol Lett 20(9):869–872
Li CX, Pan JM, Gao J, Yan YS, Zhao GQ (2009) An ion-imprinted polymer supported by attapulgite with a chitosan incorporated sol–gel process for selective separation of Ce(III). Chin Chem Lett 20(8):985–989
Sengupta A, Airan Y, Thulasidas SK, Natarajan V (2016) Appraising spectral interference of dysprosium on 27 analytes using capacitatively coupled device detector based inductively coupled plasma atomic emission spectroscopy. At Spectrosc 37(2):50–60
Perevalov SA, Malofeeva GI, Kuzovkina EV, Spivakov BY (2013) Solid-phase extraction of plutonium in various oxidation states from simulated groundwater using N-benzoylphenylhydroxylamine. J Radioanal Nucl Chem 295(1):1–6
Schiewer S, Volesky B (1995) Model in softhe proton-metal ion exchange in biosorption. Environ Sci Technol 29(12):3049–3058
Thiollet G, Musikas C (1989) Synthesis and uses of the amides extractants. Solv Extr Ion Exch 7(5):813–827
Castellan GW (1983) Physical chemistry, 3rd edn. Addison-Wesley, Boston
Duff DG, Ross SMC, Huw VD (1988) Adsorption form solution: an experiment to illustrate the langmuir isotherm. J Chem Ed 65(9):815–820
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, freundlich, temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2? unto phosphoric acid modified rice husk. J Appl Chem 3(1):38–45
Sengupta A, Jayabun SK, Pius IC, Thulasidas SK (2015) Synthesis, characterization and application of metal oxides impregnated silica for the sorption of thorium. J Radioanal Nucl Chem. doi:10.1007/s10967-015-4658-4
Mohan SV, Karthikeyan J (1997) Removal of lignin and tannin colour from aqueous solution by adsorption onto activated charcoal. Environ Pollut 97(1–2):183–187
Singh DB, Prasad G, Rupainwar DC, Singh VN (1988) As(III) removal from aqueous solution by adsorption. Water Air Soil Pollut 42(3):373–386
Sengupta A, Keskar M, Jayabun SK (2016) Sorption behaviour of metal ion on thorium tungstate synthesized by solid state route. J Radioanal Nucl Chem. doi:10.1007/s10967-016-4895-1
Sengupta A, Jayabun SK, Boda A, Ali SKM (2016) An amide functionalized task specific carbon nanotube for the sorption of tetra and hexa valent actinides: experimental and theoretical insight. RSC Adv 6(46):39553–39562
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Modi MK, Pattanaik P, Dash N, Subramanian S (2015) Sorption of Radionuclides. Int J Pharm Sci Rev Res 34(1):122–130
Dhami PS, Kannan R, Naik PW, Gopalakrishnan V, Ramanujam A, Salvi NA, Chattopadhyay S (2002) Biosorption of amercium using biomasses of various rhizopus species. Biotechnol Lett 24(11):855–889
Dutta S, Mohapatra PK, Ramnani SP, Sabharwal S, Das AK, Manchanda VK (2008) Use of chitosan derivatives as solid phase extractors for metal ions. Desalination 232(1–3):234–242
Tobin JM, Cooper DG, Neufeld RJ (1984) Uptake of metal ions by Rhizopus arrhizus biomass. Appl Environ Microbiol 47(4):821–824.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kishor, P., Adya, V.C., Sengupta, A. et al. Understanding the sorption behavior of tetra- and hexavalent plutonium on fungus Rhizopus arrhizus dead biomass. J Radioanal Nucl Chem 311, 903–912 (2017). https://doi.org/10.1007/s10967-016-5104-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5104-y