Abstract
Extraction chromatographic separations following a nuclear event will require both speed and accuracy. Ideally these separations will be carried out using commercially available products. Because 90Sr is a likely material to be used in a radioactivity dispersal device, commercially available products for the separation of strontium were tested to evaluate differences in their elution profiles that may affect elution procedures. Gravity flow columns show better resolution, but vacuum flow columns allow rapid processing and are still adequate for purification if multiple aqueous conditions can be employed in the elution strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following the terrorist attacks of the last two decades, a number of previously unconsidered threats have emerged in the minds of government authorities, including radiation dispersal devices [1]. A radiation dispersal device (RDD), or “dirty bomb”, could come in many shapes and sizes, but would effectively consist of some conventional explosive combined with radioactive material [2]. Two of the key impact factors of an RDD attack are the quantity and type of radiological material employed. Isotopes important in the discussion of RDDs include the alpha emitters 238Pu, 241Am, and 252Cf, the beta/gamma emitters 60Co, 137Cs, and 192Ir, and the pure beta emitter 90Sr [3, 4]. Taking the possible modes of acquisition a terrorist organization may employ into account, as well as factors such as the breadth of use, security, and sheer number of sources in circulation, most concerned organizations agree that 137Cs, 60Co, 192Ir, and 90Sr pose the most plausible risk to society [4]. 90Sr and its daughter 90Y are both pure beta emitters, which means that an RDD equipped with 90Sr would require significantly less shielding than a device with a similar amount of one of the other plausible nuclides mentioned above. Quantification of the radioactive material dispersed by the RDD will also be much more difficult because of the low signal available without first isolating the isotope. These factors make 90Sr an isotope of significant interest.
Quantification of 90Sr in such a situation would be an important part of accomplishing several goals, of which the primary two are (1) determination of the age of the radiostrontium source and (2) establishing the total amount of 90Sr used in the attack by comparing collected samples to measured radiation fields. There are many procedures that have been developed over the years to isolate strontium from environmental samples, and it is likely that a variation of one of these would be used in quantifying the strontium in the area. The majority of the modern methods developed employ extraction chromatography with commercially available Sr Resin, produced by Eichrom Technologies (Lisle, IL), to carry out the actual separation [5].
Extraction chromatography is a powerful and easy to use technique that was first developed nearly 50 years ago and employed slow-moving gravity flow columns [6]. Modern techniques tend to employ the same selective resins, but pack them into a ready-to-use cartridge that has solution pulled through it using a vacuum. These cartridges have been in use for several years now with the assumption that they still have the same characteristics as the gravity flow columns. This study investigates whether there are any differences between the vacuum and gravity flow elution characteristics.
Experimental
Materials
Free resin loaded with 40 % w/w of a 1.0 M 4,4′(5′)-di-t-butylcyclohexano 18-crown-6 in 1-octanol solution was obtained from Eichrom Technology in the form of their Sr-Resin. Pre-packed gravity flow columns and cartridges provided by the same vendor for use with Eichrom’s vacuum box technology were also utilized. 137Cs was obtained from Eckert & Ziegler Isotope Products in the form of cesium chloride in 0.5 M HCl. All cesium working solutions were prepared by directly taking an aliquot from the stock solution and diluting it in 0.5 M nitric acid. 85Sr was obtained from Perkin Elmer in the form of strontium chloride in 0.5 M HCl. All strontium working solutions were prepared by directly taking an aliquot from the stock solution and diluting it in 0.5 M nitric acid. Nitric acid solutions were prepared from TraceMetal grade nitric acid (Fisher Scientific) and deionized water from a NanoPure ultrapure water system (Barnstead).
Procedure
Column preparation
Three types of columns were analyzed in this work: pre-packed vacuum and gravity cartridges and slurry-packed gravity columns. Pre-packed vacuum cartridges and gravity columns were used as received from the manufacturer. Slurry packed gravity columns were prepared by slurrying a known amount of resin in 0.5 M nitric acid and quantitatively transferring it to a disposable plastic BioRad PolyPrep column (4 cm H × 0.8 cm ID with 10 mL reservoir). The resin was allowed to settle overnight, then the column was opened to allow the acid to flow through and compact the resin bed. Following this, the bed height was measured and glass wool was added to the top of the column to prevent the bed from being disturbed during the addition of sample. A small additional amount of acid was added to the column to keep the resin wet during storage and the column was sealed with two endcaps until it was used.
Column characterization
Important column parameters in extraction chromatography are identified in Markl and Schmid, and include the stationary phase volume (resin volume), bed density, bed volume and mobile phase volume (also referred to as interstitial space, void volume, or free column volume) [6]. In order to determine these values, the resin density must be known. In this case it is given by the manufacturer as 1.12 g mL−1 [7].
Because the vacuum cartridges and pre-packaged columns are sealed, the amount of resin in each had to be experimentally determined. For the vacuum columns, this was done by taking the mass of several full cartridges, splitting them open and removing all of the resin, then taking the mass of the empty cartridge. The bed volume was also determined at this time by measuring the length of the column prior to splitting it open, the height of the frit on either end of the column after splitting the column open and subtracting the two to get the bed height, measuring the inner diameter of the cartridge, and geometrically calculating the total bed volume. A similar method was used with the pre-packed gravity columns, except that the resin was also dried in an oven at 90 °C for 18 h before weighing to determine the mass of resin in each due to the presence of acid in the column. The bed volume of the pre-packed gravity columns was determined by measuring the height of the bed before emptying the column and measuring the inner diameter of the column after.
The amount of resin added to the slurry packed columns was measured prior to packing, and the inner dimensions were determined by pipetting 2 mL of water into each column and measuring the height of the water in the column. The water was then discarded and the average diameter of the column was calculated. The column was then slurry packed via the method outlined above, and the height of the bed was used in conjunction with the diameter of the column to calculate the total bed volume. For each type of column, the bed volume and mass of resin in the column was then used to determine the bed density. Finally, the free column volume (FCV) of the columns was determined by using the resin density to determine the volume of resin in each column and subtracting this from the total bed volume.
Each column was preconditioned with 4 mL of 0.5 M nitric acid, then loaded with 10 µL of cesium working solution (~2000 CPM µL−1 in 0.5 M HNO3). The FCV of each column was experimentally determined by measuring the breakthrough volume of 137Cs, which effectively has no affinity for the resin in 0.5 M nitric acid [7]. Fractions were collected in 100 µL increments.
Strontium elution
After pre-conditioning the columns with 4 mL of 0.5 M nitric acid the columns were loaded with 0.25 mL of strontium working solution (~340 CPM µL−1 in 0.5 M HNO3) and eluted with 50 FCV of 0.5 M nitric acid, which was collected in ~5 FCV increments.
Measurement
Gamma spectroscopy was performed using a WIZARD2 2480 automatic gamma counter from PerkinElmer. The instrument was equipped with a 3 inch well-type NaI(Tl) detector with a 75 mm solid lead shield for background reduction. Samples were counted for 5 min each.
Results and discussion
Column characterization
The characteristics of the slurry packed gravity flow columns are summarized in Table 1. Note that the experimentally measured free column volume values (“breakthrough volumes” found in Table 2) match up well with those calculated from geometric column parameter measurements. The larger amount of variation seen in the measured slurry packed gravity column data is a reflection of the differences in each column which, due to being prepared by hand, have much larger differences in resin packing quality than those prepared in bulk from the distributor. This becomes very evident when the elution profiles are examined. Slurry packed columns numbered one and two have fairly similar profiles, but slurry packed column number three has a much lower, broader peak that also elutes later than the first two, as can be seen in Fig. 1. This is likely due to small irregularities in the packing structure caused by allowing the flow of solution to compact the resin bed instead of using compressed gas, as suggested by previous researchers [6, 8]. Regardless of this variation, all of the average parameters still match very well with the previously published bulk and column characterization data [7].
The pre-packed gravity columns show equally good agreement with the previously published data [7]. Their parameters can also be found in Table 1, and FCV data in Table 2. It should be noted that the calculated free column volumes of the pre-packed columns are somewhat low compared to those experimentally observed by breakthrough. This is likely caused by the larger deviation in measured resin contained in the pre-packed columns. Despite this, the elution profiles are much more reproducible than the slurry packed columns, which can be seen by comparing the profiles from the slurry packed columns in Fig. 1 with those of the pre-packed columns in Fig. 2. The pre-packed columns are also slightly longer than the slurry packed columns, giving them a larger number of theoretical plates, indicating better separations would be possible given the same acid matrices.
The vacuum column cartridges, in contrast to the two gravity-type columns, show significant differences between the measured parameters determined for the columns and the previously published data [7]. The average column parameters are summarized in Table 1, with the average FCV data found in Table 2. While the amount of resin contained by the vacuum cartridges corresponds to a calculated FCV similar to that of the slurry packed gravity flow columns (~70 %), the FCV measured experimentally via breakthrough volume is much lower (~30 %). In all likelihood, the answer to why this occurs can be found in the mechanism by which these columns are operated.
The conventional use of vacuum cartridges calls for the column to be dried each time a fraction is pulled through. Since the elution band is not an infinitely thin line, there will be portions of the band farther down the column that are still in solution while portions farther up the column are already dried, making band broadening in the direction of flow much more pronounced than in traditional columns that are kept wet the duration of the elution. If an analyte is eluted that is negligibly retained by the resin (such as cesium [7]), there will therefore be significant band broadening and the breakthrough volume will actually be less than one full free column volume. However, the peak of the elution profile should still correspond to the most concentrated part of the elution band. This means that if the “un-retained” analyte could be added in a sufficiently small volume, the peak of the elution profile should correspond roughly to the FCV, regardless of the band broadening caused in the forward direction. Because the cesium was added in very small load fractions in this study, the peak locations from Fig. 3 were compiled in Table 3 and compared to the calculated average FCV from Table 2. It can be seen from this that the average peak location differs from the predicted FCV by much less than the breakthrough volume (7.9 and 51.8 % differences, respectively), indicating a much better prediction. Furthermore, this argument is strengthened by the fact that the previously published retention values agree with the elution profiles generated in this study when the peak of the cesium elution is used as the FCV instead of the breakthrough for the vacuum columns [7].
Strontium elution
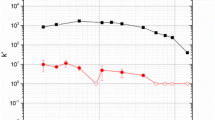
According to work previously carried out by the authors as well as data published by the developers of strontium resin, the number of FCVs to the elution peak maximum (k’) of strontium in 0.5 M nitric acid should be in the range of 12–15 free column volumes [7]. As seen in Fig. 4, both types of gravity columns and the vacuum columns show elution peaks occurring at approximately 15–18 FCV. Given that the data points are plotted at the middle of the collection volume, the size of the fractions collected (~6 mL), and the error associated with the preparation of the acid, this is in excellent agreement with the published values [7]. Both the vacuum and slurry packed columns also have effectively quantitative elution of strontium, with 95 % or more being recovered from each of the columns and an average recovery of 97.9 %. The pre-packed columns have slightly lower recovery, but still average 93.5 %. Despite these similarities, there are also significant differences in the elution profiles of the three types of columns, which can be seen in Fig. 4.
The most obvious difference in the elution profiles is the band broadening. The vacuum flow columns show significant band broadening and tailing in comparison to both types of gravity columns. While it was hypothesized that this could be explained by the much higher flow rate of the vacuum columns (~1 mL min−1 compared to ~0.2 mL min−1), results from examining this variable were mixed. Initially, a second column elution performed at ~4 mL min−1 showed that the faster flow rate resulted in a much broader elution profile, as seen in Fig. 5. An additional set of vacuum columns was also run at ~4 mL min−1 at a different facility, however, and resulted in an elution profile similar to that obtained at ~1 mL min−1 the first time. This can be seen in Fig. 6, where ANL denotes work done at Argonne National Laboratory and UNLV denotes work done at the University of Nevada, Las Vegas. While the data presented in Fig. 5 indicates that flow rate does play an important role in band broadening, the data presented in Fig. 6 shows that there are clearly also effects based on the lot of resin used in packing the cartridges. Because of this, it is difficult to draw any sound conclusions other than that, for a given lot of cartridges, higher flow rates will result in more band broadening.
The pre-packed gravity columns and the vacuum columns are much more reproducible than the slurry packed gravity columns, as evidenced by the substantially smaller error bars (representing 1σ variation) associated with these in comparison to the slurry packed columns in Fig. 4. The resolution of both the slurry packed and pre-packed columns are much better than the vacuum columns (and could likely be better with smaller fraction sizes), with more than 80 % of their activity being concentrated in two fractions instead of up to six, as is the case with the vacuum columns.
One interesting trend that becomes apparent from this study is that, while the geometric and experimentally measured free column volumes correspond to ~70 % of the total bed volume for each of the different columns, the manufactured columns are smaller than the 2 mL advertised volume. This is especially true of the vacuum columns, which are a full 0.4 mL short. Subsequent experimentation in this study, however, shows that in practice the published retention values are accurate when the free column volume is assumed to be 1.4 mL instead of the experimentally determined FCV. This is evidenced by elution profiles consistently having peaks in the 15–23 FCV range when using the measured FCV instead of the factory specified value. If the listed FCV is applied, these peaks all shift down by approximately three FCVs, making the range 12–20, slightly more in line with the published values of 12–15 FCV [7].
Conclusion
While both types of gravity flow columns appear to have better resolution than the vacuum flow columns, the massive difference in speed and the ability to measure out fractions before eluting them makes vacuum columns still the more desirable separation method for processing purposes. The vacuum columns also do not require constant attention, can be handled without having to worry about disturbing the bed, and the resolution can (likely) be improved by using elution strategies that utilize high retention load and rinse fractions followed by extremely low retention stripping fractions. If a situation requires a chromatographic separation without changing the elution matrix, however, it would be a better choice to use some form of gravity flow column.
References
Andersson KG, Mikkelsen T, Astrup P, Thykier-Nielsen S, Jacobson LH, Hoe SC, Nielsen SP (2009) Requirements for estimation of doses from contaminants dispersed by a ‘Dirty Bomb’ explosion in an urban area. J Environ Radioact 100:1005–1011
Elcock D, Klemic GA, Taboas AL (2004) Establishing remediation levels in response to a radiological dispersal event (or “Dirty Bomb”). Environ Sci Technol 38(9):2505–2512
Parra RR, Medina VF, Conca JL (2009) The use of fixatives for response to a radiation dispersal devise attack—a review of the current (2009) state-of-the-art. J Environ Radioact 100(11):923–934
Ferguson CD, Kazi T, Perera J (2003) Commercial radioactive sources: surveying the security risks. Center for Nonproliferation Studies, Monterey Institute of International Studies, Monterey
Vajda N, Kim C-K (2010) Determination of radiostrontium isotopes: a review of analytical methodology. Appl Radiat Isot 68(12):2306–2326
Markl P, Schmid ER (1975) Techniques in column extraction chromatography. In: Braun T, Ghersini G (eds) Extraction chromatography. Elselvier, New York, p 45
Horwitz EP, Chiarizia R, Dietz ML (1992) A novel strontium-selective extraction chromatographic resin. Solvent Extr Ion Exch 10(2):313–336
Horwitz EP, Bloomquist CAA (1972) The preparation, performance and factors affecting band spreading of high efficiency extraction chromatographic columns for actinide separations. J Inorg Nucl Chem 34:3851–3871
Acknowledgments
This material is based upon work supported by the U.S. Department of Homeland Security under Grant Award Number, 2012-DN-130-NF0001. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the U.S. Department of Homeland Security.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McLain, D.R., Mertz, C.J. & Sudowe, R. A performance comparison of commercially available strontium extraction chromatography columns. J Radioanal Nucl Chem 307, 1825–1831 (2016). https://doi.org/10.1007/s10967-015-4634-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4634-z