Abstract
In the present study, radon concentrations were measured in 70 groundwater samples collected from Tuticorin district of Tamil Nadu, India. Radon activity concentration was found in the range of 0.07–40.70 Bq L−1 with an average value of 5.13 Bq L−1. Radon values were compared with USEPA and WHO. In this study radon activity trend is above the permissible limit may be due to influence of heavy rainfall. A good correlation is found with deeper borehole depth and lithology. Granitic terrain is found to have higher radon concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radon (222Rn) is a naturally occurring radioactive gas formed from the decaying uranium and radium deposits, having a half-life of 3.823 days and emits radioactive alpha particles. It escapes from the earth’s crust through cracks and crevices in bedrock and dissolves in groundwater. The radon content in groundwater depends on the radium concentration in the rock of the aquifer [1, 2]. Radon concentrations are dominantly controlled by geological factors [3, 4], with limited secure relationship to depth of water level and radon concentration [5].
Groundwater drawn from granitic and metamorphic rocks frequently presents relatively high radioactivity, mostly due to the presence of uranium, radium and radon isotopes. In particular, the radon isotope (222Rn) seems to have particular significance since it is normally much enriched in groundwater in relation to other uranium series isotopes and is in large disequilibrium with its 226Ra parent isotope. Furthermore, radon is one of the main agents of radioactivity transfer from crust’s uppermost layers to lower atmosphere. There are published reports on the presence of radon in groundwater drawn from granitic and metamorphic terrains [6, 7]. Radon content in groundwater in these terrains are quite variable and in part reflects the aquifer rock type. Generally, the 222Rn concentrations in water are regulated, the maximum contaminant levels (MCL) has been proposed by the US Environmental Protection Agency (USEPA) (11.3 Bq L−1) [8] and WHO [9] proposed a potable water level for human consumption (100 Bq L−1). The exposure of population to high concentrations of radon and its daughters for a long period lead to pathological effects like the respiratory functional changes and the occurrence of lung cancer [10]. Also, a very high level of radon in drinking water can also lead to a significant risk of stomach and gastrointestinal cancer [11]. In India, the radon concentrations of groundwater in different states are given the Table 1. The aim of the present study is to investigate the radon concentrations in groundwater used for drinking purpose, its distribution in groundwater and to understand the correlation between the radon concentrations and geology, well depth etc. in the Tuticorin district of Tamil nadu State, India.
Study area
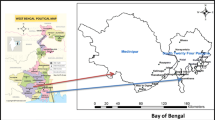
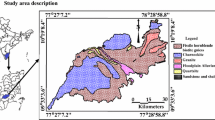
Tuticorin district is situated in southern part of Tamil nadu, the largest and driest state of India (Fig. 1). It lies between 8°19′ to 9°22′ North latitude and 77°40′ to 78°23′ East longitude encompassing a geographical area of about 4621 km2. The southwest monsoon rainfall is highly erratic and summer rains are negligible. The normal annual rainfall over the district varies from about 570 to 740 mm with a hot tropical climate. The major rivers namely, Vaipar, Tambraparani and Karamanaiyar drain the area. They flow towards the east and finally join the Gulf of Mannar. The Vembar, Chittar, Malatter and Kallar are the tributaries drains in the study area. All the rivers are ephemeral in nature and the runoff is generated by heavy rainfall events only [12]. The study area is chiefly composed of hornblende biotite gneiss, alluvial marine and charnockites in the west. The quartzite formations are also found as disseminated patches in the study area. There are minor representation of Cretaceous formation and granitic intrusion in the eastern part of the study area (Fig. 1). Major water bearing formations are Quaternary Alluvium, Tertiary sediments, and Teri sands [12, 13] weathered and fractured Pink Granites, Charnockites and Gneisses.
Sampling and measurement
Radon measurement in groundwater
A total of 70 groundwater samples were collected from hand pumps and bore wells of different depths and from various lithological units in the study area, [Alluvial Plain (13), Charnockite (3), Fluvial Plain (7), Granite (2), Hornblende Biotite Gneiss (HBG) (40) and Quartzite (5)] (Fig. 1) for the measurement of radon in groundwater, during southwest monsoon season (2014). The water samples were taken in 250-ml glass bottles. The bore well was flushed to ensure that the collected water is fresh from the aquifer without the chance of gas escape. The water was collected using a small diameter polythene tube in order to avoid turbulence and through exposure of water samples to the atmosphere. The collected samples were sent to the laboratory and analysed on the same day. Depth of the bore well, year of construction of the well and time of sample collection and measurement were recorded. The location map is shown in Fig. 1.
In the laboratory, the dissolved radon is stripped out from water by bubbling air and circulated through a closed air-loop via a desiccant tube into the 222Rn counting system. The air recirculates through the water and continuously extracts the radon until a state of equilibrium develops. The system reaches this state of equilibrium within 5 min and more than 95 % of the available radon is removed from the water during this time. The purpose of the desiccant is to absorb moisture, since detection efficiency decreases at higher humidity. The 222Rn activity is then counted for 40 min (4 cycles of counting) after attaining equilibrium.
RAD-7 uses a high electric field above a silicon semiconductor detector at ground potential to attract the positively charged polonium daughters, 218Po+ (half life = 3.1 min; alpha energy = 6.00 MeV) and 214Po+ (half life = 164 µs; alpha energy = 7.67 MeV), which are counted as a measure of 222Rn concentration in air. An air filter at the inlet of the radon monitor prevents dust particles and charged ions from entering into the alpha detector. The ions are collected in energy specific windows which eliminate interference and maintain very low background. 222Rn activities are expressed in Bq m−3 (disintegration per second per m3) with 2σ uncertainties. All 222Rn activities are corrected for the radioactive decay with respect to the sampling time.
Un-acidified sub-samples were collected from the same 70 locations in 500-ml bottles for cation and anion analysis. Physical parameters such as pH, temperature, TDS and electrical conductivity (EC) of the water samples were measured in the field using a portable water-analysis kit. In the laboratory, Na+, Ca2+, Mg2+, K+, Cl−, HCO3 −, SO4 2−, NO3 −, PO4 3− and F− were analyzed by Ion Chromatography. Duplicate samples were analysed to check the analytical precision; it is ±5 % for all hydrogeochemical variables.
Results and discussion
Water chemistry
Groundwater in the study area is generally alkaline in nature with pH ranging from 6.90 to 9.40 with an average of 7.80 (Table 2). The temperature ranges from 28 to 32 °C with an average of 29 °C in the study area. EC is an indirect measure of ionic strength and mineralization of natural water. EC ranges from to 343 to 13,265 μS/cm with an average of 2190 μS/cm. Total dissolved solids (TDS) is the sum of dissolved ionic concentration that varies between 220 and 8490 mg L−1 with an average of 1398 mg L−1.
The order of dominance of cations in the study area is as follows Na+>Ca2+>Mg2+>K+ (all values are in mg L−1). Na+ concentration vary from 12 to 2930 mg L−1 with an average of 223 mg L−1. Ca2+ concentration ranges from 32 to 500 mg L−1 with an average of 115 mg L−1. The concentration of magnesium in groundwater samples in the study area varies from 9 to 895 mg L−1 with an average of 106 mg L−1. K+ concentration ranges from 0.40 to 520 mg L−1, with an average of 40 mg L−1. The order of dominance of Anions in the study area is follows Cl−>HCO3 −>SO4 2−>NO3 −>F− > PO4 3− (all values are in mg L−1). Chloride ranges from 35 to 9052 mg L−1 with an average of 694 mg L−1. Bicarbonate ranges from 85 to 683 mg L−1 with an average of 284 mg L−1. In groundwater samples, SO4 2− concentration varies from 4 to 312 mg L−1 with an average of 77 mg L−1. The concentration of NO3 − varies from 0.84 to 148.20 mg L−1 with an average of 10.28 mg L−1. The concentration of F− varies from Below Detectable Limit (BDL) to 2.42 mg L−1 with an average of 0.48 mg L−1. The concentration of PO4 3− varies from BDL to 1 mg L−1 with an average of 0.16 mg L−1.
Hydrogeochemical facies
Hill Piper plot [14] is used to infer hydrogeochemical facies of groundwater (Fig. 2). In alluvial plain, samples are clustered in the fields of 1, 2, 4 and 5, and the majority of the samples are concentrated in the Ca2+–HCO3 − type (Fig. 2), indicating the dominance of freshwater recharge into the aquifers [15]. A few samples fall on Na+–Cl− types, indicating the saline nature of groundwater that may be due to proximity to the coastal region [16]. Other samples with minor representations from mixed Ca2+–Mg2+–Cl−, Ca2+–Cl−, and Ca2+–HCO3 − types. From the plot, alkalis (Na+ and K+) exceed alkaline earths (Ca2+ and Mg2+) and strong acids (Cl− and SO4 2−) exceed weak acid (HCO3 −). Most of the samples from the Charnockite terrain fall in field 5 and they are mainly Ca2+–Cl− facies. Groundwater samples from the granite region mainly fall in the fields of 4. Samples from the quartzite region also fall in the same field, and most of them are mixed Ca2+–Mg2+–Cl− facies indicating the dominance of reverse ion-exchange waters. HBG samples are clustered in the fields of 1, 2, 4 and 5, and the majority of the samples are concentrated in the Ca2+–Mg2+–Cl− facies, indicating dominance of reverse ion-exchange waters. In fluvial plain, the dominant fields are 2, 4 and 5, and the majority of the samples are concentrated in Na+–Cl− facies indicating the influence of seawater intrusion along the coastal region. A few samples fall on Ca2+–Mg2+–Cl− facies indicating the dominance of reverse ion-exchange waters and Ca2+–Cl− facies may be a leading edge of the seawater intrusion [17]. From the plot, strong seawater influence is evident in fluvial plain region which indicate a clear shift from Ca2+–Cl− to Na+–Cl− type through Ca2+–Mg2+–Cl− facies [18].
Radon concentration in groundwater
The measured radon concentration in groundwater samples collected from Tuticorin district of Tamil Nadu is given in Fig. 3. The 222Rn concentrations in the samples were in the range of 0.07–40.70 Bq L−1 with an average value of 5.13 Bq L−1. The USEPA has proposed that the allowed MCL for radon concentration in water is 11.3 Bq L−1 [8]. The WHO has suggested a value of radon concentration in potable water is 100 Bq L−1 [9]. In the present study area, it is seen that 10 samples (Ramanachiyarpuram, Pudiyampuththur, Melasekkarakudi, Thiruchendur, Naduvakuruchi, Kulathur, Chennampatti, Alagapuri, Palayakayal, and Punnakayal) sample falls above the permissible limit (Fig. 3) according to USEPA [8] and all the sample fall on potable water range according to WHO [9]. It is also noted that most of the groundwater samples fall above the permissible limit in the study area is due to the influence of highly rainfall [19], similar trend is observed in Himalaya region [20]. When the measured radon concentration values were compared with the European Commission recommendations on the protection of the public against exposure to radon in drinking water supplies, which recommends the action level of 100 Bq L−1 for public water supplies [21], all the recorded values were found to be well below the action level and hence safe for drinking purposes.
Variation of radon concentration with depth
The data were analyzed to find out correlation between the depths of bore wells and the radon contents. It is observed that generally very shallow wells have low concentration of radon, as seen in dug well water at Aathur (Sample number: 5) having a depth of 3 m with a radon concentration of 0.88 Bq L−1 in alluvial region. The bore well at Thiruchendur (sample number: 7) having a depth of 24 m has radon content of 12.92 Bq L−1 in fluvial region, while at Pudukottai (sample number: 55) the bore well having a depth of 110 m is having a concentration of 9.04 Bq L−1 in quartzite region. The bore well at Puthiamputhur (sample number: 18) having a depth of 122 m has radon content of 11.91 Bq L−1 in charnockite region. The highest concentration of 40.70 Bq L−1 (granite region) is reported in Punnakayal (sample number: 63) bore well, 183 m deep, while the deepest bore well (245 m) at Alagapuri (sample number: 26) is having radon content of 24.42 Bq L−1 in HBG. Similar trend found out the strong correlation between depth to water level and radon concentration [22]. The lithology-wise radon concentration and depth of water level is presented in Table 3.
Less significant correlation between the radon concentration and depth in alluvial and fluvial plains are as a result of unconsolidated sediments. The large number of pores in the unconsolidated sediments helps the produced radon to escape into the atmosphere through these pores (Fig. 4). Frequent recharge takes place in this formation indicating the atmospheric interaction. Majority of the samples in hard rock terrain such as granite, charnockite, quartzite and HBG shows an increase in radon with depth. At deeper depths, the rock is compact and the pores, cracks, cavities etc. decreases, because of the confined nature and solubility of radon in water increases the radon concentration at deeper depths [6].
Correlation between radon concentration and lithology
Lithology and radon concentration in groundwater shows good correlation in the study area. Figure 5 depicts the spatial variation of radon concentrations in groundwater. The lithology-wise categorization of the radon concentration in groundwater shows that higher concentration was observed in the granitic terrain followed by charnockite (Table 3). Radon shows a strong lithological dependence (Fig. 5). The highest concentration of radon was (40.70 Bq L−1) observed in the granitic rocks, along the southeastern part of the study area. Granite shows higher radon concentration when compared with other formations. Similar observation of high values of radon is reported in granite and sheared gneisses [23, 24]. Box and Whisker plots were used to explain the distribution of the above data sets (Fig. 6). A clear decrease in median values of radon is noted as follows: Granite > Charnockite > Hornblende Biotite Gneiss > Quartzite > Alluvial plain > Fluvial plain.
Radon with temperature, pH, total dissolved solid (TDS)
Temperature is an important factor that affects the radon content in the groundwater (Fig. 7a). The concentration of 222Rn in a water sample is considered using the well-known temperature-dependent distribution factor of 222Rn between air and water [25]. Minimum temperature was observed in granite rock type (28.1 °C) and highest temperature was observed in alluvial region (31 °C) respectively. The highest radon concentration (40.77 Bq L−1) resembles the lowest temperature and lowest concentration of radon of 0.88 Bq L−1 shows the highest values of temperature in the groundwater samples. Majority of the groundwater sample shows that radon solubility in water decreases with increasing temperature [26].
222Rn concentrations plotted as function of pH (Fig. 7b). When pH (6.9) decreases highest radon concentration is observed in the groundwater samples and lowest radon concentration resembles with higher value of pH (7.5) in study area. The distribution of data points on the plots presented show no linear correlation and these results agree with similar studies performed elsewhere [27] as these factors may also influence the radon in the groundwater. The lowest radon concentration has the TDS value of 1025 ppm in fluvial plain regions and highest radon concentration has the TDS value of 1690 ppm (Fig. 7c). These results are in agreement with similar observation made in groundwater studies in Italy [28].
Conclusion
From the study, it is concluded that:
-
1.
The order of dominance of cations and anions in the groundwater are Na+>Ca2+>Mg2+>K+ and Cl−>HCO3 −>SO4 2−>NO3 −>F−> PO4 3− respectively.
-
2.
From piper plot, strong seawater influence is evident in fluvial plain region which indicate a clear shift from Ca2+–Cl− to Na+–Cl− type through Ca2+–Mg2+–Cl− facies.
-
3.
The radon concentrations in groundwater samples collected from the Tuticorin district were ranges from 0.07–40.70 Bq L−1 with an average value of 5.13 Bq L−1.
-
4.
The recorded values of radon concentration in groundwater are exceeding the safe limit recommended by the USEPA.
-
5.
Most of radon concentration in groundwater samples of the study area are above the permissible limit due to influence of heavy rainfall during study period.
-
6.
There is a linear relation between the radon concentration and the depth of bore wells. Lithologically radon concentration indicates that granite is having the maximum radon concentrations. However, it is observed that the groundwater from the shallow aquifers is having the least concentration of radon, as its escape to the atmosphere.
-
7.
It is observed that there is a good correlation exist between the presence of high radon content and the presence of granitic and HBG rocks. The peripheral areas of granites and HBG also have higher concentration of radon and it is a function of degree of weathering.
-
8.
Spatial distribution of radon in groundwater shows a gradual increase of radon concentration towards the southeastern part of the study area represented by granitic intrusions.
-
9.
The order of dominance of the radon in groundwater sample representing the follows: Granite > Charnockite > Hornblende Biotite Gneiss > Quartzite > Alluvia plain > Fluvial plain.
-
10.
The highest radon concentration (40.77 Bq L−1) observed in granitic rock with lowest temperature and lowest concentration of radon of 0.88 Bq L−1 in the alluvial region with higher values of temperature.
However, detailed studies are essential for further confirming the health hazards and seasonal variation of radon concentration in groundwater in the area.
References
Prasad G, Prasad Y, Gusain GS, Ramola RC (2009) Radiat Meas 43:S375–S379
Shivakumara BC, Chandrashekara MS, Kavitha E, Paramesh L (2014) J Radiat Res Appl Sci 7:491–498
Przylibski TA, Gorecka J (2014) J Environ Radioact 134:43–53
Krishan G, Rao MS, Kumar CP, Semwal P (2015) J Radioanal Nucl Chem 303(3):2221–2225
Przylibski TA (2011) Nat Hazards Earth Syst Sci 11:1695–1703
Thivya C, Chidambaram S, Tirumalesh K, Prasanna MV, Thilagavathi R, Nepolian M (2015) J Radioanal Nucl Chem 302:1349–1355
Mirhosseini SM, Negarestani A, Moattar F, Karbassi AR (2015) J Radioanal Nucl Chem 304(3):1085–1092
USEPA (1991) Federal Register. 40 CFR parts 141 and 142 National Primary Drinking Water Regulations, Radionuclides: Proposed Rule. Government Printing office, Washington
WHO (2004) Guidelines for drinking water quality, 3rd edn. WHO, Geneva
Kendal GM, Smith TJ (2002) J Radiol Prot 22:389–406
Rani A, Mehra R, Duggal V (2013) Radiat Prot Dosimety 153:496–501
CGWB (Central Ground Water Board) (2009) South Eastern Coastal Region, District groundwater brochure, Thoothukudi District
Singaraja C, Chidambaram S, Anandhan P, Prasanna MV, Thivya C, Thilagavathi R, Sarathidasan J (2013) Environ Dev Sustain 16:689–721
Piper AM (1953) A graphic procedure I the geo-chemical interpretation of water analysis, USGS groundwater note no, 12
Singaraja C, Chidambaram S, Noble Jacob, Ezhilarasan E, Velmurugan C, Manikandan M, Rajamani S (2015) Environ Dev Sustain 10.1007/s10668-015-9654-x
Singaraja C, Chidambaram S, Anandhan P, Prasanna MV, Thivya C, Thilagavathi R, Sarathidasan J (2013) Arab J Geosci. doi:10.1007/s12517-012-0794-0
Jeen SK, Kim JM, Ko KS, Yum B, Chang HW (2001) Geosci J 5:339–348
Rasouli FS, Masoudi SF (2012) Iran J Med Phys 9(3):183–192
Singaraja C, Chidambaram S, Anandhan P, Prasann MV, Thivya C, Thilagavathi R, Sarathidasan J (2014) Appl Water Sci 4:241–250
Choubey VM, Arora BR, Barbosa SM (2011) Naresh Kumar, Leena Kamra. J Appl Radiat Iso 69:1070–1078
European Commission (2001) Commission recommendation of 20th December 2001 on the protection of the public against exposure to radon in drinking water. 2001/982/Euratom, L344/85. Official Journal of the European Commission
Ravikumar P, Deljo D, Sharika M, Somashekar RK, Prakash KL (2014) Jour Geol Soc India 83(2):156
Selvasekarapandian S, Sivakumar R, Manikandan NM, Ragjunath VM, Kannan V, Rajaram S (2002) J Radioanal Nucl Chem 252(2):345–347
Somashekar R, Ravikumar P (2010) J Radioanal Nucl Chem 285:343–351
Dulaiova H, Camilli R, Henderson PB, Charette MA (2010) J Environ Radioact 101:553–563
Segovia N, Mena M, Monnin M, Pena P, Salazar S, Seidel JL, Tamez E (1997) Radiat Meas 28(1–6):741–744
Amrani D, Cherouati DE, Cherchal MEH (2000) J Environ Radioact 51:173–180
D’Alessandro W, Vita F (2003) J Environ Radioact 65:187–201
Najeeb K, Vinayachandran N, Jose B, Vashistha R (2014) J Geol Soc India 83:665–668
Keesari T, Mohokar HV, Sahoo BK, Mallesh G (2014) J Radioanal Nucl Chem 302:1391–1398
Duggal V, Mehra R, Rani A (2013) Radiat Prot Environ 36(2):65–70
Duggal V, Mehra R, Rani A (2013) Radiat Prot Dosimety 156(2):239–245
Duggal V, Rani A, Mehra R (2012) Adv Appl Sci Res 3:3825–3830
Bodhan K, Mehra R, Sonkawade RG (2010) Indian J Pure Appl Phys 48:508–511
Hunse TM, Najeeb KMD, Rajarajan K, Muthukkannan M (2010) J Geol Soc India 75:704–708
Prasad Y, Prasad G, Choubey VM, Ramola RC (2009) Radiat Meas 44:122–126
Vinay Kumar Reddy K, Sreenivasa Reddy B, Sreenath Reddy M, Gopal Reddy C, Yadagiri Reddy P, Rama Reddy K (2003) Radiat Meas 36:419–423
Choubey VM, Bartarya SK, Ramola RC (2003) Radiat Meas 36:401–405
Virk HS, Walia V, Bajwa BS (2001) Indian J Pure Appl Phys 39:746–749
Choubey VM, Ramola RC (1997) Environ Geol 32:258–262
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singaraja, C., Chidambaram, S., Jacob, N. et al. Radon levels in groundwater in the Tuticorin district of Tamil Nadu, South India. J Radioanal Nucl Chem 307, 1165–1173 (2016). https://doi.org/10.1007/s10967-015-4312-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4312-1