Abstract
The biosportion of Th(IV) by the marine-derived Fungus Fusarium sp. #ZZF51 was study. The Biosorption was found to be at a maximum (79.24 %), in a solution containing 50 mg Th/L, at pH 5.0, with 0.28 g dry biomass. The Temkin isotherm model and pseudo-second-order kinetic model was found to fit the data very well over the entire range of concentrations. The FTIR analysis reveals that the carboxyl, amino and hydroxyl groups on the cell wall of Fusarium sp. #ZZF51 play an important role in Th(IV) biosorption process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thorium is a naturally radioactive element and it distributes widely in earth’s crust with various forms. Tetravalent thorium(IV) is the main form in nature, and it is also useful as a tracer when studying environmentally important process [1–4]. The toxic nature of this radionuclide, even at trace level, has been a public health problem for many years. When thorium(IV) ions enter into the living organisms, it accumulates in lung, liver, spleen and marrow [5]. In the past few decades, although some techniques were available for the removal of thorium(IV) ions from aqueous solutions including chemical precipitation, electrofloatation, ion exchange, reverse osmosis and adsorption [6], they generally suffered from some disadvantages such as high operational cost, limited tolerance to pH change, incomplete metal removal, high cost of reagents, and energy requirements [7–9]. Therefore, the above methods have been restricted in application.
At present, biosorption by using microorganism especially for the living mycelium has been seen as a potential technology for removal of toxic heavy metals from industrial water, which main advantages are the reusability of biomaterial, low operating cost, easy fermentation cultivation, the improved selectivity for specific metals, short operation time, no toxic production of secondary compounds and so on [10–14]. It is well known that marine environment has its special properties such as high pressure, low temperature, less light, high salt and poor nutrition, which determine marine-derived microorganisms have theirs own special species and metabolic approaches beneficial to remove heavy metal ions. Mangrove endophytic fungus Fusarium sp. #ZZF51 was collected from South China Sea (Zhanjiang sea area). The initial work showed that the fungus could produce special metabolites and had strong ability to uptake copper(II), uranium(VI) and thorium(IV) ions by non-living biomass [15–17]. In order to systematically research the biosorption of the tested fungus so that it can be truly applied in practice in the future, this paper studies the adsorption models and the optimized conditions for thorium(IV) onto the living tested fungus, and the adsorption mechanism is also investigated.
Materials and methods
Materials
All used reagents are analytical grade unless otherwise stated. 1 g L−1 of thorium(IV) stock solution was prepared by dissolving 2.3795 g Th(NO3)4·4H2O in 20 mL of 7 mol L−1 HCl and being diluted with distilled water to 1 L. The working solutions with different concentration were obtained by diluting the stock solution to appropriate volumes. Arsenazo-III solution was prepared by dissolving 0.1250 g arsenazo-III in 5 mL nitric acid with pH 3.0 and being diluted with distilled water to 250 mL, and the pH value was adjusted by 0.1 mol L−1 HCl and NaOH solutions.
Preparation of the biosorption materials
Fussarium sp. #ZZF51 was obtained from the South China Sea coast (Zhanjiang sea area) and deposited in school of chemistry and chemical engineering, Sun Yat-sen (Zhongshan) University, Guangzhou, China. At pH 7.0 and temperature 25 °C, the tested fungus was grown in liquid medium GPY that was consisted of glucose 10 g L−1, sea salt 2 g L−1, peptone 2 g L−1 and yeast extract 1 g L−1 [17]. 22 days later, the living biomass was harvested and washed two more times with distilled water, then it was spin-dried by high-speed centrifuge and promptly used in later experiments to ensure the tested mycelium live in the whole adsorption course.
Biosorption experiments
The batch biosorption experiments were carried out at different pH values, initial metal concentration and contact time under the same conditions of temperature 25 °C, living biomass dosage 3.0 g in 50 mL solution and agitation rate 150 rpm. In fact, 3.0 g living biomass dosage could become 0.28 g dry non-living mycelium after the disposal process of being dried at 120 °C for 5 h. In the batch adsorption experiments, 3.0 g living biomass dosage was added to 50 mL thorium(IV) solution in 250 mL flask which pH value was controlled within 2.0–10.0 and concentration 20–60 mg L−1. The flask was shaken for different time (0–24 h), then the aqueous sample was centrifuged for 10 min at 4000 rpm. The thorium(IV) concentration in supernatant was determined by UV visible spectrophotometer at 660 nm through the thorium(IV) arsenazo(III) complex method [18]. All the experiments were carried out independently in triplicate. The biosorption capacity and thorium(IV) removal efficiency by mycelium were respectively calculated by using the following equations [Eqs. (1) and (2)]:
where C 0 and C e are the initial and equilibrium thorium(IV) concentrations (mg L−1), respectively. m (g) is the mass of mycelium, and V(L) is the volume of solution.
SEM and FTIR spectroscopy analysis
The surface characters of Fusarium sp. #ZZF51 biomass including before and after loaded by thorium(IV) ions were analyzed using scanning electron microscopy (SEM). The timed Fusarium sp. #ZZF51 myceliums were placed on cover slip and air-dried, then the samples were fixed in a 2.5 % glutaraldehyde solution for 7 h, and they were dehydrated 20 min in the graded ethanol series (30, 50, 70, 95 and 100 % ethanol). Finally, the samples were air-dried and sputter-coated with 4 nm of gold particles before using [19].
Fourier transformed infrared (FTIR) spectrometry were used to probe the functional groups in the mycelium of the tested fungus. Sample disks were made by mixing 5 mg of dry non-living mycelium with 150 mg of KBr. Data analysis was focused on the 400–4000 cm−1 region.
Results and discussion
FTIR spectra characterization of biomass
FTIR spectra are helpful to identify functional groups in the tested materials [20]. The FTIR spectra of the virgin and thorium(IV)-loaded biosorbents are respectively shown in Fig. 1a, b. As seen in Fig. 1a, the broad and strong band ranging from 3200 to 3600 cm−1 may be attributed to –OH groups and –NH2 groups, and the band at 2924 cm−1 is due to C–H stretching vibrations. The strong peak at 1639 cm−1 is produced by –C=O stretching vibration. Compared with FTIR spectrum of virgin biosorbent, FTIR spectrum of thorium(IV)-loaded roots in Fig. 1b displays significant shifts in some peaks. The FTIR spectroscopic analysis of thorium(IV)-loaded biosorbents indicates absorption peak site and intensity shift for asymmetrical stretching band at 3278 cm−1 (indicative of –OH and –NH groups) when compared with that of unloaded biomass which shows the same type of absorption at 3462 cm−1. This is a typical red shift phenomenon as the result of thorium(IV) ions bonding the related functional groups in the tested biomass. Another shift of the peak from 1639 to 1654 cm−1 reflects the change in the stretching frequency of carboxylate when linking thorium(IV) ions. Above observations indicate the involvement of functional groups –OH, –NH, and –C=O in the whole biosorption process.
Scanning electron microscopy (SEM)
To further investigate the adsorption process, the native and thorium(IV)-loaded biomass are observed under the Scanning electron microscopy, and the SEM images of the tested biomass are shown in Fig. 2. The image of the virgin biomass (Fig. 2a) shows a regular symmetry structure in the material surface, and amounts of small pores can be also seen, which indicates that this material presents good characteristics to be employed as a natural adsorbent for metallic ions uptake, as previously reported [21], while the surface of thorium(IV)-loaded biomass appears a rough asymmetry structure as seen in Fig. 2b. These observations denote that the tested sample especially in the virgin biomass can provide ready access and rich surface area for thorium(IV) ions onto the binding sites.
Effect of pH on biosorption
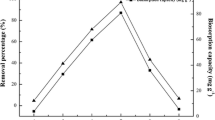
It has been shown that the affinity of cationic species towards the functional groups present in the surface is strongly dependent on the pH value in solution [12]. Figure 3 describes the relation of pH values and the adsorption of thorium(IV) ions by living mycelium. The batch biosorption experiments results show thorium(IV) uptake is significantly affected by pH, and the optimal biosorption efficiency is observed at pH 5.0, which is obviously higher than the pH 3.0 of thorium(IV) maximum biosorption efficiency for the non-living biomass of fungus Fusarium sp. #ZZF51 [15]. This can be explained as the result of living mycelium fond of the mildly acidic environment for thorium(IV) adsorption. When pH is between 4.0 and 5.0, thorium(IV) ions biosorption increases with the decrease of hydrogen ions competing effect. At pH value 5.0, the biosorption efficiency attains maximum. With further increase in pH values, thorium(IV) hydroxides such as Th(OH)3+, Th(OH2 2+), Th2 \( ({\text{OH}}_{2}^{6 + } ) \), Th6 \( ({\text{OH}}_{15}^{9 + } ) \) and so on start forming, which leads to the decline of thorium(IV) percentage removal and biosorption efficiency [22].
Effect of contact time on biosorption
In general, the adsorbed metal ions tend to desorb and go back into the aqueous solution in the late of adsorption process. Figure 4 illustrates the effect of time on thorium(IV) biosorption. It was clear that large amount of sorbent sites are vacant and the thorium(IV) concentration gradient is high at the beginning of biosorption. After 8 h, the change of sorption efficiency for thorium(IV) does not show notable effects, and this may be because of the metal binding sites saturation. Therefore, it can be concluded that the equilibrium reachs at 8 h, which is much longer than the thorium(IV) removal time 20 min for the non-living mycelium of fungus Fusarium sp. #ZZF51 [15]. The data obtained from the experiments are successfully evaluated by the following kinetics models.
Effect of biosorbent dosage on biosorption
Biosorbent dose also is an important parameter, which can influence the biosorption capacity and efficiency. The effect of adsorbent dosage on thorium(IV) removal is shown in Fig. 5. When biomass dose increases from 1.0 g to 3.0 g, thorium(IV) sorption efficiency increases significantly, which mainly owes to the increase of active sites in biomass, so the metal ions can easily go onto the adsorption sites. When the adsorbent dosage exceeds 3.0 g, the percentage removal of thorium(IV) ions decreases, and it can be explained that high dosage material produces a ‘screen effect’ on the cell wall, which protects the binding sites [23].
Effect of initial thorium(IV) concentration on biosorption
The other parameters such as pH value, solution volume and contact time are kept the above optimal values, and the effect of initial thorium(IV) concentration varying from 20 to 60 mg L−1 on biosorption by the tested living mycelium is shown in Fig. 6. It is clear that the sorption capacity increases when the initial thorium(IV) concentration increases from 30 to 50 mg L−1, and the sorption capacity slightly decreases when the initial concentration is over 50 mg L−1. The maximum biosorption capacity for the living biomass is 6.96 mg g−1, which is less than that (11.35 mg g−1) for the non-living mycelium [15]. The maximum biosorption capacity should come at the moment of available free sites (metal binding sites) approaching saturation at higher thorium(IV) concentration. From the sorption characteristic, it can be perceived that the surface saturation of sorbents is dependent on the concentration of initial thorium(IV) solution, and the binding sites of the sorbents are regarded as constant [24].
Adsorption kinetics
Kinetic study can provide valuable information for the mechanism of adsorption and the optimum operating conditions of industrial-scale batch process. In the study, the pseudo-first-order, pseudo-second-order and Elovich kinetic models are used to interpret the experimental kinetic data [25], and their rate equations are represented as follows:
where q e (mg g−1) and q t (mg g−1) are the amounts of thorium(IV) adsorbed at equilibrium time and t (min), respectively. k 1 (min−1) and k 2 (g mg−1 min−1) are the rate constants for the pseudo-first-order and pseudo-second-order equation.
where α is the initial sorption rate (mg g−1 min−1), and β denotes the activation energy for chemisorption (g mg−1).
The values of q e, k 1, k 2, α, β are calculated according to the slopes and the intercepts of straight lines, and the relevent experiment data are presented in Table 1. It is obvious that the correlation coefficient (R 2 = 0.9990) for the pseudo-second-order model is higher compared with those of the pseudo-first-order adsorption model and Elovich model. So Lagergren’s pseudo-second-order kinetic model can adequately fit the adsorption data better for the tested sorbent, which indicates that the adsorption process involves the course of chemical sorption in addition to physical rate-limiting step.
Biosorption isotherm
In order to optimize the biosorption process, it is necessary to obtain the appropriate correlation for equilibrium curve. In this study, Langmuir, Freundlich and Temkin adsorption isotherms are used to linear fitting with the experimental data [14]. The Langmuir isotherm equation is valid for monolayer sorption onto surface containing finite number of identical sorption sites, which is described by the following equation:
where Q (mg g−1) represents the amount of adsorption and b (L mg−1) denotes the sorption equilibrium constant, respectively.
The Freundlich equation is purely and empirically based on sorption of heterogeneous surface, which is commonly presented as:
where k is the constant of sorption capacity, and 1/n represents the constant of sorption intensity.
where A and B are Temkin isotherm constants, R is the universal gas constant (8.314 J mol−1 K−1), T represents absolute temperature, C e is on behalf of the residual equilibrium concentration (mg L−1), and q e denotes the amount of adsorbent (mg g−1) at equilibrium. Experimental data are modeled according to Langumir, Freundlich, Temkin isotherms, and the evaluated constants are presented in Table 2. In view of the correlation regression coefficients, the adsorption process can be better described by Temkin model (R 2 = 0.998) and Langumir model (R 2 = 0.997) than Freundlich model (R 2 = 0.987). Consequently, it could be concluded that the biosorption of live biosorbents might follow a special sorption model, and other mechanisms such as intracellular bioaccumulation would contribute to the uptake of thorium(IV) except for surface binding.
Conclusion
Batch adsorption experiments exhibit that the living mycelium of fungus Fusarium sp. #ZZF51 has a good ability for thorium(IV) biosorption. The tested sample can provide ready access and rich surface area for thorium(IV) onto the binding sites by SEM analysis. Hydroxyl, amino, and carbonyl groups have an important contribution to the adsorption process by comparing with Fourier transform infrared spectra for the tested fungus before and after loaded with thorium(IV). Thorium(IV) ions onto the living fungus mycelium was optimized at pH 5.0, equilibrium time 8 h, thorium(IV) initial concentration 50 mg L−1 and living biomass dosage 3.0 g in 50 mL solution with 79.24 % of removal efficiency and 6.96 mg g−1 of sorption capacity, which is less than that (11.35 mg g−1) of the non-living mycelium for thorium(IV) biosorption under its optimized condition. The experimental data are analyzed by using classical isotherm models and kinetic equations, and it is obtained that the Temkin isotherm model and the pseudo-second-order kinetic model provide better correlation with the experimental data for biosorption of thorium(IV).
References
Basu H, Singhal RK, Pimple MV, Manisha V, Bassan MKT, Reddy AVR, Mukherjee T (2011) Development of naturally occurring siliceous material for the preferential removal of thorium from U–Th from aquatic environment. J Radioanal Nucl Chem 289:231–237
Shtangeeva I (2010) Uptake of uranium and thorium by native and cultivated plants. J Environ Radioact 101:458–463
Gumrah O, Malcik N, Caglar P (2009) Optical ligand–thorium complex sensors using various reagents and the comparison of formation constants obtained in dip probe, flow cell and microchip systems. Sens Actuators B 139:125–131
Mahani MK, Divsar F, Chaloosi M, Maragheh MG, Khanchi AR, Rofouei MK (2008) Simultaneous determination of thorium and uranyl ions by optode spectra and chemometric techniques. Sens Actuators B 133:632–637
Kuber CB, Stanislaus FD (2009) Thorium biosorption by Aspergillus fumigatus, a filamentous fungal biomass. J Hazard Mater 165:670–676
Hritcu D, Humelnicu D, Dodi G (2012) Magnetic chitosan composite particles: evaluation of thorium and uranyl ion adsorption from aqueous solutions. Carbohydr Polym 87:1185–1191
Munagapati VS, Gutha Y, Yarramuthi V (2011) Equilibrium, kinetic and thermodynamic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by fungus (Trametes versicolor) biomass. J Taiwan Inst Chem Eng 42:965–971
Tong KS, Kassim MJ, Azraa A (2011) Adsorption of copper ion from its aqueous solution by a novel biosorbent Uncaria gambir: equilibrium, kinetics, and thermodynamic studies. Chem Eng J 170:145–153
Ghasemi M, Keshtkar AR, Dabbagh R, Safdari SJ (2011) Biosorption of uranium(VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga: breakthrough curves studies and modeling. J Hazard Mater 189:141–149
Levent A, Ahmet K, Hasan K, Mustafa II (2011) Adsorption of Cr(VI) on ureolytic mixed culture from biocatalytic calcification reactor. Colloids Surf B Biointerfaces 86:404–408
Mohammad F, Ali D, Habibollah Y (2009) Biosorption equilibria of binary Cd (II) and Ni (II) systems onto Saccharomyces cerevisiae and Ralstonia eutropha cells: application of response surface methodology. J Hazard Mater 168:1437–1448
Gabr RM, Hassan SHA, Shoreit AAM (2008) Biosorption of lead and nickel by living and non-living cells of Pseudomonas aeruginosa ASU 6a. Int Biodeterior Biodegrad 62:195–203
Nadavala SK, Kim M (2011) Phenolic compounds biosorption onto Schizophyllum commune fungus: FTIR analysis, kinetics and adsorption isotherms modeling. Chem Eng J 168:562–571
Isik M (2008) Biosorption of Ni(II) from aqueous solutions by living and non-living ureolytic mixed culture. Colloids Surf B Biointerfaces 62:97–104
Yang SK, Tan N, Yan XM, Chen F, Lin YC (2013) Adsorption of thorium(IV) from aqueous solution by non-living biomass of mangrove endophytic fungus Fusarium sp. #ZZF51. J Radioanal Nucl Chem 298:827–833
Chen F, Tan N, Long W, Yang SK, She ZG, Lin YC (2014) Enhancement of uranium(VI) biosorption by chemically modified marine-derived mangrove endophytic fungus Fusarium sp. #ZZF51. J Radioanal Nucl Chem 299:193–201
Yang HB, Tan N, Wu FJ, Liu HJ, Sun M, She ZG, Lin YC (2012) Biosorption of uranium (VI) by a mangrove endophytic fungus Fusarium sp. #ZZF51 from the South China Sea. J Radioanal Nucl Chem 292:1011–1016
Bayyari MA, Nazal MK, Khalili FI (2010) The effect of ionic strength on the extraction of thorium(IV) from perchlorate solution by didodecylphosphoric acid (HDDPA). Arab J Chem 3:115–119
Liu MX, Dong FQ, Yan XY, Zeng WM, Hou LY, Pang XF (2010) Biosorption of uranium by Saccharomyces cerevisiae and surface interactions under culture conditions. Bioresour Technol 101:8573–8580
Li N, Bai R (2005) A novel amine-shielded surface cross-linking of chitosan hydrogel beads for enhanced metal adsorption performance. Ind Eng Chem Res 44:6692–6700
Ofomaja AE, Naidoo EB (2010) Biosorption of lead(II) onto pine cone powder: studies on biosorption performance and process design to minimize biosorbent mass. Carbohydr Polym 82:1031–1042
Misaelides P, Godelitsas A, Filippidis A (1995) Thorium and uranium uptake by natural zeolitic materials. Sci Total Environ 173(174):237–246
Yang SK, Tan N, Yan XM, Chen F, Long W, Lin YC (2013) Thorium(IV) removal from aqueous medium by citric acid treated mangrove endophytic fungus Fusarium sp. #ZZF51. Mar Pollut Bull 74:213–219
Zhu B, Fan TX, Zhang D (2008) Adsorption of copper ions from aqueous solution by citric acid modified soybean straw. J Hazard Mater 153:300–308
Akar ST, Gorgulu A, Akar T (2011) Decolorization of Reactive Blue 49 contaminated solutions by Capsicum annuum seeds: batch and continuous mode biosorption applications. Chem Eng J 168:125–133
Acknowledgments
The authors wish to thank the Natural Science Foundation of China (No.20072058) and Science and Technology Development Project of Hunan (No. 2010FJ3014) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S.K., Tan, N., Wu, W.L. et al. Biosorption of thorium(IV) from aqueous solution by living biomass of marine-derived fungus Fusarium sp. #ZZF51. J Radioanal Nucl Chem 306, 99–105 (2015). https://doi.org/10.1007/s10967-015-4060-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4060-2









