Abstract
Solvent extraction of Am(III), Pu(IV) and Eu(III) was done from nitric acid medium using a solvent system containing a substituted dipicolinamide extractant dissolved in room temperature ionic liquid. The solvent system was found to be more efficient as compared to the previously reported solvents in molecular diluents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Room temperature ionic liquids (RTILs), due to their versatile and tunable physico-chemical properties have been found wide applications in separation science and technology. They have been received increased attention in industrial liquid–liquid extraction process applications as diluents, pertaining to their unique extraction behaviour for metal ions [1–4]. Attractive properties such as insignificant vapour pressure, ability to dissolve a wide range of organic and inorganic compounds, versatile electrochemical window, and tunability of physicochemical properties by suitable combination of cation and anion, etc. as possessed by RTILs [5, 6] make RTILs an interesting class of diluents and detailed investigations as alternatives to molecular diluents has been explored [7–12]. Such features of RTILs make them promising candidates for applications in nuclear fuel cycle to selectively target metal ions with interesting extraction mechanisms [13–21]. In relevance to that, many extractants and extraction processes have been evaluated in the ionic liquid media with detailed mechanistic evaluation. Even though, the evaluation processes of these proposed extractant systems are still under way, the solvent extraction studies have led to some very interesting observations [22].
High-level waste (HLW), generated after the reprocessing of the spent nuclear fuels poses major environmental threats due to long-lived radionuclides content with high radiotoxicities [23, 24]. For the remediation of the long-term hazards associated with HLW, ‘Partitioning and Transmutation’ (P & T) is the proposed strategy, which suggests partitioning of the minor actinides followed by their transmutation [25, 26]. The separation of the radioactive minor actinides and lanthanides is therefore one of the current challenges in nuclear waste reprocessing. Chemical similarities between the two groups of elements make such separations rather difficult [27]. However, actinides possess relatively more covalent character in comparison to the lanthanides, in metal–ligand bonding suggesting that ligands with soft donor atoms like N, S can exploit this small but significant difference between the actinides and lanthanides [28]. Soft N donors like tridentate aromatic bases have been found as efficient extractants for An(III)/Ln(III) separations [29]. From acidic aqueous solution, nitrogen polyaromatic ligands (with the exceptions of BTP and BTBP ligands [29–31] ) are generally too basic to extract actinides (the competition with protonation is not favorable) and they must be used with a co-extractant at low acidity [30–32]. In this context, mixed N, O donor ligands consisting of two distinct functional units, including a terpyridine moiety with soft N donors for inducing An(III)/Ln(III) selectivity at lower acidity and two amide functional groups to improve the extraction of lighter actinides (U, Np, Pu) from a highly acidic medium were proposed, in recent literature [33].
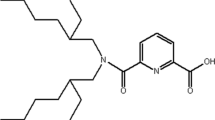
Earlier, diamides of malonic acid have been proposed as promising extractants for minor actinides [34, 35 ]. They have stimulated the studies on diglycolamides which extract minor actinides and lanthanides with very high extraction in common organic diluents [36, 37]. Reports suggested the main advantage of diamides of dipicolinic acid (DPA) ligands, as higher extraction of An(III) than of Ln(III) over other conventional diamides [38]. The Am/Eu separation factor up to 6 have been achieved using the picolinamide based systems [39–42] and have been reported to be influenced by the nature of the diluent. The type of substituent also influences the solubility of the picolinamide ligand and its metal solvate, solvent loading capacity, hydrodynamic properties and consequently the solvates’ extraction properties [43]. Detailed studies on the structural correlation for DPA have been reported earlier in polar molecular diluents [38, 39]. However, their extraction ability for trivalent actinides and lanthanides is extremely weak at higher nitric acid concentrations [44]. In order to increase Ln/An separation factors and extraction of actinide ions by N,N′-diethyl-N,N′-di(para)fluorophenyl-2,6-dipicolinamide (DEtD(p)FPhDPA, DPA) (Fig. 1a), there is a need to optimize the solvent extraction system. Our previous studies with molecular diluents have shown that the nature of the aqueous phase acid has a remarkable influence on the extraction behavior of Am(III) by the picolinamide [45]. Such interesting observations provide impetus for the extensive studies of picolinamide class of extractants in a ionic liquid medium (Fig. 1b). Furthermore, in view of synergistic effects reported in the presence of chlorinated cobalt dicarbollide (CCD, Fig. 1c), the present study also involved CCD along with DPA. To our knowledge, there is no report on the extraction of trivalent and tetravalent actinide ions using solvents containing dipicolinamides in ionic liquids. The only publication on U extraction has appeared very recently [46].
The objective of the present paper is to carry out a systematic study on the extraction of the actinides ions, Am(III), Pu(IV) and the lanthanide ion, Eu(III) from nitric acid medium using a novel ionic liquid based solvent system containing DPA. The effects of parameters such as kinetics, aqueous phase acidity (0.001–3 M HNO3), extractant concentration, and synergistic effect of hydrogen dicarbollyl cobaltate (H+CCD−) on extraction of metal ions were studied. The stoichiometry of the extracted Am(III) and Eu(III) bearing species have been evaluated from nitric acid medium. Mechanistic rationalization towards the Pu(IV) extraction, has been explained from experimentally observed extractive behavior.
Experimental
Materials and reagents
N,N′-diethyl-N,N′-di(p)-fluorophenyl dipicolinamide was synthesized from anhydride of dipicolinic acid and respective N-ethyl-(p)-fluoroaniline and provided by Khlopin Radium Institute, Russia [39]. Cesium dichlorocarbollyl cobaltate (KatChem, Czech Republic) was converted to H+CCD− by shaking several times with 4 M HClO4. Desired weights of diamides were dissolved in different RTILs ([Cnmim][NTf2], n = 4, 6, 8) (IoLiTech, Germany) by extended ultrasonication (few hours). It may be mentioned here that the ligand was stable to the sonication treatment.
Radiotracers
Purified Pu (principally 239Pu), 152,154Eu, and 241Am were used from laboratory stock solutions for the extraction experiments. Pu was purified using HTTA (2-thenoyltrifluoroacetone) extraction after adjusting its valency to the +4 state by the addition of 0.05 M NaNO2 + 0.005 M NH4VO3 to the aqueous phase (1 M HNO3). The extracted Pu(IV) was stripped using 8 M HNO3 and the resultant solution was used as the stock solution The radiochemical purity of the product was ascertained by alpha and gamma ray spectrometry using Si surface barrier and HPGe detectors, respectively.
Distribution studies
Equal volumes of organic solutions of the dipicolinamide in RTILs and aqueous phases were equilibrated, at room temperature (24 ± 1 °C) for 2 h unless stated otherwise. The two phases were then separated by centrifugation and equal volumes from both the phases was drawn for radioactivity counting. Distribution ratio of the metal ions (D M) is defined as the ratio of concentration of the respective metal ions (expressed in terms of radioactivity) in the organic phase to that in the aqueous phase. The assay of Pu samples was carried out by liquid scintillation counting (Hidex, Finland) using toluene based scintillator cocktail (SRL, Mumbai) and that of 152,154Eu, and 241Am was done by gamma ray counting using NaI(Tl) scintillator counter (Para Electronics) coupled to a multi-channel analyzer (ECIL, India). The material balance and reproducibility of the data was within the error limits of ±5 %. For all the metal ions, it was checked that the extraction of the metal ion was negligible without the dipicolinamide extractant in all the ionic liquids used in the present study.
Results and discussion
Extraction kinetics of DPA in RTIL
It was important to evaluate Am(III) extraction kinetics using DPA in the ionic liquid ([C4mim][Tf2N]) media which usually show slower kinetics of extraction as reported by Sengupta et al. for [C8mim][PF6] with a higher dynamic viscosity of 694 mPa.s leading to attainment of equilibrium after 3 h [47]. Results from the present studies showed that 30 min were sufficient for achieving equilibrium Am(III) extraction from 0.01 M HNO3 (Fig. 2) suggesting that the kinetics of extraction was relatively faster compared to those previously reported in the RTIL based solvent systems [47]. However, faster attainment of equilibrium was reported in molecular diluents [48]. This equilibrium attainment time was shorter than other RTIL based solvent systems where as long as 60 min were needed for the attainment of equilibrium [47]. It is expected that the extraction behaviour in other ionic liquids such as [C6mim][Tf2N] and [C8mim][Tf2N] are similar and in view of better extraction behaviour reported with [C4mim][Tf2N], major part of the studies are limited to this ionic liquid [49].
Evaluation of different ionic liquids
Ionic liquid with different alkyl chains show widely different extraction properties [5]. Different RTILs of general formula [Cnmim][Tf2N] (1-alkyl-3-methylimidazoliumbis(trifluoromethanesulfonyl)imide) viz. [C4mim][Tf2N], [C6mim][Tf2N], [C8mim][Tf2N] (Fig. 1b) were evaluated as the diluents for Am(III) and Eu(III) extraction from 0.01 M HNO3 while keeping DPA concentration at 0.02 M. Increasing alkyl chain length of the ionic liquid moiety from n-butyl to n-hexyl group showed a sharp decrease in Am(III) and Eu(III) extraction which subsequently was not affected significantly while using n-octyl group (Table 1). This has been attributed to the decreased hydrophilicity of imidazolium cation with increased alkyl chain length from n-butyl to n-hexyl, though the reason for no further decrease upon moving from n-hexyl to n-octyl group is not understood. The decrease in D values with increasing alkyl chain length in the cationic part of the ionic liquids is well reported [22]. The separation factor [ratio of the D values of Am(III) and Eu(III)] values are calculated to be 1.64, 3.3 and 2.55 with [C4mim][NTf2], [C6mim][NTf2], [C8mim][NTf2], respectively indicating no regular trend.
During the extraction of Sr(II), Eu(III), Am(III) and U(VI) from HNO3 media using crown ethers, CMPO [octyl-(phenyl)–N,N-diisobutyl carbamoyl methyl phosphine oxide], tripodal diglycolamide (T-DGA), Tributyl phosphate (TBP), tetraoctyl diglycolamides (TODGA) and malonamides as ligands; similar observations have been reported elsewhere [15–20]. It is worth noting that significantly lower concentration of DPA (0.02 M) offers high extraction of Am(III) and Eu(II) compared to 0.5–1 M diamide/picolinamides concentration generally used in polar molecular diluents [45]. This indicates that by fine tuning the cationic substituent’s of RTIL, it may be possible to perform actinide partitioning experiments using much lower concentration of the DPA. Due to better extraction properties by [C4mim][NTf2], it was used for all the subsequent experiments.
Effect of the aqueous phase acidity
D M values decreased with increased nitric acid concentration implying that at higher acidities H+ competes with the metal cations as per the well known cation-exchange mechanism operative in case of ionic liquids such as [C4mim][Tf2N] as shown in Eq. (1) and is well reported in the literature [15, 22, 50].
where M = Am(III) or Eu(III), L = DPA, and subscripts ‘aq’ and ‘IL’ refer to aqueous and ionic liquid phases, respectively (Fig. 3). This effect was less pronounced in case of [C8mim][Tf2N] due to its less hydrophilicity making cation exchange less probable (Fig. 3). In view of not so different extraction behaviour of [C6mim][Tf2N] as compared to [C8mim][Tf2N] (Table 1), analogous studies in this diluent medium was not undertaken. It was observed that the extractability decreased considerably in [C8mim][Tf2N] as compared to the [C4mim][Tf2N] extraction system which may be due to changes in the extraction mechanism. This conclusion was based on a less steep decrease in Am(III)/Eu(III) extraction with increasing HNO3 concentration. In contrast, D Am increased with nitric acid concentration in molecular diluent medium suggesting solvation mechanism during the extraction process for malonamides and dipicolinamides [45]. Similar decreasing trend for Am(III) extraction for malonamides as the extractants was attributed to the interaction of ligand molecules with nitric acid concentration [35, 47]. The above results have two consequences. Firstly, the separation factor values are close to unity suggesting not very effective for the metal ions. Secondly, due to lower extraction of the metal ions at higher acidities, the metal ion stripping can be carried out at higher concentration of HNO3 (6–8 M). However, as quantitative stripping was not possible in a single stage, several stages may be required for this purpose.
Stoichiometry of the extracted species
In polar diluents, dipicolinamide derivatives showed mono-solvated extracted species from 3 M HNO3 aqueous phase [48]. Nature of the ligand, diluent and the aqueous phase acid concentration significantly influences the nature of the extracted species [35]. Therefore, it was interesting to know the nature of the extracted species formed by DPA in ionic liquid mediated extraction process. The DPA concentration variation experiments carried out keeping the nitric acid concentration constant at 0.01 M indicated that possibly 2–3 ligand molecules are present in the extracted species [slope = 2.52 ± 0.05 for Am(III); slope = 2.47 ± 0.03 for Eu(III)] in [C4mim][NTf2] from 0.01 M HNO3 aqueous phase (Fig. 4). This suggests that the extraction equilibrium can be shown as follows in Eq. (2):
where M = Am or Eu, L = DPA, and ‘x’ is close to 2.5 suggesting the extraction of mixed extraction species of the type 1:2 and 1:3 (M:L). Such mixed extraction species are not uncommon and have been reported in ionic liquid medium as well [47].
Effect of dicarbollide on Am(III) and Eu(III) extraction
Hydrogen dichlorocarbollylcobaltate (H+CCD−) is an important synergistic extractant, especially so in the case of DPA ligands [45] and acts as counter anion to improve the extractability. Due to its bulkier size, it gives significant hydrophobicity to the metal–ligand complex and facilitates extraction process. At higher acidities, the decrease in the extraction is observed due to the replacement of CCD− by the hydrophilic NO3 − anion in the extracted species [45]. It is worth mentioning that DPA in molecular diluents showed an enhancement in the extraction with increasing aqueous phase acidities. By contrast, for DPA/CCD mixture display opposite trend and was attributed to the protonation of the pyridine ring nitrogen to deplete the availability of the electron pair and complexation of NO3 − ions to metal ions making extracted species less lipophilic as compared to CCD− anion at higher acidities. Therefore, it was of interest to study the effect of [H+CCD−] on trivalent metal ion extraction in ionic liquid medium. As shown in Table 1, the presence of CCD significantly enhances the extractability of both Am(III) and Eu(III) metal ions due to more hydrophobic extracted species. However, there was no significant change in the separation factors of both the trivalent metal ions.
Comparative extraction of Am(III) and Eu(III)
In recent literature, DPA in polar fluorinated medium FS-3 has been used to achieve the maximum separation factors for the actinide lanthanides separations [38]. Irrespective of this advantage, the poor extractability provides the impetus to optimize the extractant system to achieve the maximum extraction. As shown in Table 2, the use of bulkier synergistic extractant like CCD− improves the extractability but decreases the S.F. (separation factor) value to 2 from the molecular diluents like FS-13. However, the DPA dissolved in the ionic liquid diluents increases the extraction performance manifold albeit with decreased An(III)/Ln(III) separation efficiency. This comparison implies that in extracted species of picolinamides like DPA dissolved in molecular diluents, the actinides showed higher covalent character than the lanthanides. This results in the better S.F. with lesser D M values. However, in CCD based synergistic system, the bulkier counter anion gives more lipophilic character to increase the solubility of the extracted species in organic phase and increasing the extraction efficacy [45]. In case of the RTIL based system, the better solubility of DPA as well as its extracted species with Am(III) and Eu(III) gave far better extractability. The decreased S.F. can be attributed to the cation exchange mechanism, which diminishes the effect of slight co-ordination differences between the Am/Ln [45]. It is worth mentioning here that other soft donor ligands such as Cyanex 301, BTP, BTBP, etc. result in much improved separation behaviour.
Pu(IV) extraction by DPA in [C4mim][NTf2]
Though trivalent lanthanides and actinides have been reported to be extracted using RTIL based solvent systems, analogous studies involving the tetravalent actinide ions such as Pu(IV) were less explored. It was, therefore, of interest to investigate the extraction behaviour of Pu(IV) in the solvent system containing DPA in RTIL. For DPA dissolved in RTIL ([C4mim][NTf2]), the extraction of Pu(IV) increased with increasing acidities (Fig. 5), which is opposite to that of the trivalent metal ions extraction of the other metal ions such as Am(III) and Eu(III). In earlier literature on malonamides in RTIL, extraction of Pu(IV) was reported from higher concentration of HNO3 (>4 M) involving anion exchange mechanism without participation of the malonamide in the extracted species [45]. In case of DPA in RTIL, no extraction of Pu(IV) in the absence of the ligand was observed. Bonnaffe-Moity et al. have reported that anionic species of U(VI) with malonamides get extracted by anion exchange mechanism with NTf2 − [21]. At the given acidity (0.5–4 M), such mechanism is unfavorable due to very low possibility of anionic complexes of Pu(IV). Therefore, the observed increase in D Pu can be explained by a cation mechanism containing NO3 − ions in the extracted species, analogous to that reported by us [22], as given in Eq. (3) [22]:
where L = DPA, and subscripts ‘aq’ and ‘IL’ refer to aqueous and ionic liquid phases, respectively.
Conclusions
The DPA/RTIL based system has been evaluated as an alternative to the hazardous fluorinated diluents based extraction processes. The extractability for all the metal ions under study [Am(III), Eu(III) and Pu(IV)] increased multifold even with very low concentrations of picolinamide. Whereas the extraction of Am(III) and Eu(III) decreased with increased aqueous phase acidity; Pu(IV) extraction showed an opposite trend. Both these observations have been explained in terms of ion exchange mechanism which is observed in many analogous ionic liquid based solvent systems containing neutral donor ligands [1, 5]. The extraction of trivalent metal ions is proposed to take place without the involvement of nitrate ions, on the other hand, the nitrated species of Pu(IV) is extracted by cation exchange mechanism using DPA dissolved in [C4mim] [NTf2]. However, the separation factors values for Am(III)/Eu(III) are not encouraging as compared to that in molecular diluents having solvation mechanism. The RTILs can be studied extensively in view of the good solubility, which is the major difficulty in picolinamide extraction studies.
References
Nakashima K, Kubota F, Maruyama T, Goto M (2005) Feasibility of ionic liquids as alternative separation media for industrial solvent extraction processes. Ind Eng Chem Res 44:4368–4372
Ajioka T, Oshima S, Hirayama N (2008) Use of 8-sulfonamidoquinoline derivatives as chelate extraction reagents in ionic liquid extraction system. Talanta 74:903–908
Sun X, Luo H, Dai S (2012) Solvent extraction of rare-earth ions based on functionalized ionic liquids. Talanta 90:132–137
Wei G, Yang Z, Chen C (2003) Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal Chim Acta 488:183–192
Vasudeva Rao PR, Venkatesan KA, Rout A, Srinivasan TG, Nagarajan K (2012) Potential applications of room temperature ionic liquids for fission products and actinide separation. Sep Sci Technol 47:204–222
Billard I, Ouadi A, Gaillard C (2011) Liquid–liquid extraction of actinides, lanthanides, and fission products by use of ionic liquids: from discovery to understanding. Anal Bioanal Chem 400:1555–1566
Cocalia VA, Gutowski KE, Rogers RD (2006) The coordination chemistry of actinides in ionic liquids: a review of experiment and simulation. Coord Chem Rev 250:755–764
Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2009) A review on the eletrochemical applications of room temperature ionic liquids in nuclear fuel cycle. J Nucl Radiochem Sci 10:R1–R6
Binnemans K (2007) Lanthanides and actinides in ionic liquids. Chem Rev 107:2592–2614
Rout A, Binnemans K (2014) Liquid–liquid extraction of europium(III) and other trivalent rare-earth ions using a non-fluorinated functionalized ionic liquid. Dalton Trans 43:1862–1872
Rout A, Wellens S, Binnemans K (2014) Extraction of rare earths and nickel by solvent extraction with two mutually immiscible ionic liquids. RSC Adv 43:5753–5768
Mudring AV, Tang S (2010) Ionic liquids for lanthanide and actinide chemistry. Eur J Inorg Chem 41:2569–2581
Visser AE, Swatloski RP, Griffin ST, Hartman DH, Rogers RD (2001) Liquid/liquid extraction of metal ions in room temperature ionic liquids. Sep Sci Technol 36:785–804
Dai S, Barnes CE, Ju YH (1999) Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids. J Chem Soc, Dalton Trans 8:1201–1202
Dietz ML, Dzielawa JA (2001) Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: implications for the ‘greenness’ of ionic liquids as diluents in liquid–liquid extraction. Chem Commun 20:2124–2125
Dietz ML, Dzielawa JA, Laszak I, Young BA, Jensen MP (2003) Influence of solvent structural variations on the mechanism of facilitated ion transfer into room-temperature ionic liquids. Green Chem 5:682–685
Rout A, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2011) Room temperature ionic liquid diluent for the extraction of Eu(III) using TRUEX extractants. J Radioanal Nucl Chem 290:215–219
Sengupta A, Mohapatra PK, Iqbal M, Verboom W, Huskens J, Godbole SV (2012) Extraction of Am(III) using novel solvent systems containing a tripodal diglycolamide ligand in room temperature ionic liquids: a ‘green’ approach for radioactive waste processing. RSC Adv 2:7492–7500
Billard I, Ouadi A, Jobin E, Champion J, Gaillard C, Georg S (2011) Understanding the extraction mechanism in ionic liquids: UO 2+/2 HNO3/TBP/C4-mimTf2N as a case study. Solv Extr Ion Exch 29:577–601
Shen Y, Tan X, Wang L, Wu W (2011) Extraction of the uranyl ion from the aqueous phase into an ionic liquid by diglycolamide. Sep Purif Technol 78:298–302
Bonnaffé-Moity M, Ouadi A, Mazan V, Miroshnichenko S, Ternova D, Georg S, Sypula M, Gaillard C, Billard I (2012) Comparison of uranyl extraction mechanisms in an ionic liquid by use of malonamide or malonamide-functionalized ionic liquid. Dalton Trans 41:7526–7536
Patil AB, Pathak PN, Shinde VS, Godbole SV, Mohapatra PK (2013) Efficient solvent system containing malonamides in room temperature ionic liquids: actinide extraction, fluorescence and radiolytic degradation studies. Dalton Trans 42:1519–1529
Mathur JN, Murali MS, Nash KL (2001) Actinide partitioning—a review. Solv Extr Ion Exch 19:357–390
Status and trends of spent fuel reprocessing (1999) IAEA TECDOC-1103
Lewis FW, Harwood LM, Hudson MJ, Drew MGB, Desreux JF, Vidick G, Bouslimani N, Modolo G, Wilden A, Sypula MN, Vu TH, Simonin JP (2011) Highly efficient separation of actinides from lanthanides by a phenanthroline-derived bis-triazine ligand. J Am Chem Soc 133:13093–13103
Magill J, Berthou V, Haas D, Galy J, Schenkel R, Wiese HW, Heusener G, Tommasi J, Youinou G (2003) Impact limits o partitioning and transmutation scenarios on the radiotoxicity of actinides in radioactive waste. Nucl Energy 42:263–277
Nash KL, Madic C, Mathur JN, Lacquement J (2006) Actinide separation science and technology. In: Morss LR., Edelstein NM, Fuger J and Katz JJ (eds.)The chemistry of the actinide and transactinide element, 3rd edn, vol 4, Springer, The Netherlands, p 2622
Choppin GR (2002) Covalency in f-element bonds. J Alloys Compd 344:55–59
Kolarik Z (2008) Complexation and separation of lanthanides(III) and actinides(III) by heterocyclic N-donors in solutions. Chem Rev 108:4208–4252
Hill C, Berthon L, Guillaneux D, Dancausse JP, Madic C, (2004) Proceedings of Atalante 2004, Nîmes, France
Kolarik Z, Müllich U, Gassner F (1999) Selective extraction of Am(III) over Eu(III) by 2,6-ditriazolyl- and 2,6-ditriazinylpyridines. Solv Extr Ion Exch 17:23–32
Kolarik Z, Müllich U, Gassner F (1999) Extraction of Am(III) and Eu(III) nitrates by 2,6-di-(5,6-dipropyl-1,2,4-triazin-3-yl)pyridines. Solv Extr Ion Exch 17:1155–1170
Marie C, Miguirditchian M, Guillaneux D, Bisson J, Pipelier M, Dubreuil D (2011) New Bitopic ligands for the group actinide separation by solvent extraction. Solv Extr Ion Exch 29:292–315
Madic C, Hudson MJ (1998) European report EUR. 18038
Patil AB, Shinde VS, Pathak PN, Mohapatra PK, Manchanda VK (2013) Modified synthesis scheme for N, N′-dimethyl-N, N′-dioctyl-2, (2′-hexyloxyethyl) malonamide (DMDOHEMA) and its comparison with proposed solvents for actinide partitioning. Radiochim Acta 101:93–100
Sasaki Y, Tachimori S (2002) Extraction of actinides(III), (IV), (V), (VI), and lanthanides(III) by structurally tailored diamides. Solv Extr Ion Exch 20:21–34
Ansari SA, Pathak PN, Mohapatra PK, Manchanda VK (2012) Chemistry of diglycolamides: promising extractants for actinide partitioning. Chem Rev 112:1751–1772
Alyapyshev MY, Babain VA, Tkachenko LI, Eliseev II, Didenko V, Petrov ML (2011) Dependence of extraction properties of 2,6-dicarboxypyridine diamides on extractant structure. Solv Extr Ion Exch 29:619–636
Mowafy EA, Shalash AM, El-Nagar IM (2003) Extraction of certain radionuclides by bipicolinamides as new extractants from nitric acid medium. Ind J Chem A 42:3012–3016
Mowafy EA (2007) Evaluation of selectivity and radiolysis behavior of some promising isonicotinamids and dipicolinamides as extractants. Radiochim Acta 95:539–545
Alyapyshev MY, Babain VA, Antonov NG, Smirnov IV (2006) Extraction of americium and europium from perchloric acid solutions with N, N′-dialkyl-and N, N, N′, N′-tetraalkylpyridine-2,6- dicarboxam. Russ J Appl Chem 79:1808–1815
Alyapyshev MY, Babain VA, Smirnov IV, Shadrin AYu (2006) Separation of americium and europium from solutions of nitric and perchloric acid using dipicolinic acid diamides. Czech J Phys 56:D469–D475
Babain VA, Alyapyshev MY, Kiseleva RN (2007) Metal extraction by N, N′-dialkyl-N, N′-diaryl-dipicolinamides from nitric acid solution. Radiochim Acta 95:217–223
Babain VA, Alyapyshev MY, Smirnov IV, Shadrin AYu (2006) Extraction of Am and Eu with N, N′-substituted pyridine-2,6- dicarboxamides in fluorinated diluent. Radiochemistry 48:369–373
Patil AB, Pathak PN, Shinde VS, Alyapyshev MY, Babain VA, Mohapatra PK (2014) A novel dipicolinamide-dicarbollide synergistic solvent system for actinide extraction. Radiochim Acta 102:481–487
Sun M, Yuan LY, Tan Y, Zhao YL, Chai ZF, Shi WQ (2014) Solvent extraction of uranium(VI) by a dipicolinamide using a room-temperature ionic liquid. Radiochim Acta 102:87–92
Sengupta A, Mohapatra PK, Iqbal M, Huskens J, Verboom W (2012) A highly efficient solvent system containing functionalized diglycolamides and an ionic liquid for americium recovery from radioactive wastes. Dalton Trans 41:6970–6979
Panja S, Mohapatra PK, Tripathi SC, Gandhi PM, Janardan P (2012) A highly efficient solvent system containing TODGA in room temperature ionic liquids for actinide extraction. Sep Purif Technol 96:289–295
Sun X, Luo H, Dai S (2012) Ionic liquids based extraction: a promising strategy for the advanced nuclear fuel cycle. Chem Rev 112:2100–2128
Makrlík E, Vaňura P, Selucký P, Babain VA, Smirnov IV (2009) Extractive distribution of microamounts of europium and americium in the two phase water—HCl—nitrobenzene—N, N, N′, N′-tetraethyl-2,6-dipicolinamide—hydrogen dicarbollyl cobaltate system. J Radioanal Nucl Chem 279:743–747
Acknowledgments
The authors (PNP and PKM) thank Dr. A. Goswami, Head, Radiochemistry Division for his keen interest in this work. ABP greatfully acknowledges the financial support for his fellowship from Bhabha Atomic Research Centre, Mumbai, under BARC-University of Pune, Pune Collaborative Research Programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patil, A.B., Pathak, P.N., Shinde, V.S. et al. A novel solvent system containing a dipicolinamide in room temperature ionic liquids for actinide ion extraction. J Radioanal Nucl Chem 305, 521–528 (2015). https://doi.org/10.1007/s10967-015-4028-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4028-2