Abstract
To verifying the feasibility of uranium recovery with fungal metabolic products in large-scale applications, column leaching and ion exchange of uranium was carried out. The uranium recovery reached 81.76 % in 14 days. The ion exchange curve for the leach solution obtained with the metabolic products of Aspergillus niger was in the shape of a wave. The elution curve was similar to that of leaching with H2SO4. The results indicate that leaching with the metabolic products of A. niger is a promising and environmentally friendly method for exploitation of low grade uranium ores in large-scale applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is one of the strategic elements and is used as a fuel in nuclear power plants. The application of microorganisms in the recovery of uranium can effectively reduce investment cost, improve uranium recovery and shorten the leaching cycle [1, 2]. Bacterial leaching of uranium with chemoautotrophic bacteria has been extensively studied, including Acidithiobacillus ferroxidans, Acidithiobacillus thiooxidans, Acidthiobacillus caldus, Leptospirillum ferrooxidans, and so on [3, 4]. These microorganisms utilize iron and sulfur as substrate and carbon dioxide as carbon source [5, 6]. The substrate utilization way indicates that the bacteria are only suitable for the leaching of sulfide ores. For chemoautotrophic bacteria grow in an acidic environment, the acidification pretreatment is required for the leaching. For the leaching with A. ferroxidans, the pH of the ore pulp has to be adjusted to two before A. ferroxidans is inoculated [7], and uranium recovery can be above 70 % after the acidification [8]. This indicates that A. ferroxidans just plays an auxiliary role in leaching. In addition, its growth is slow, and its biomass is small. All these disadvantages limit the application of chemoautotroph bacteria in uranium leaching.
Many fungi are able to produce organic acids such as citric and oxalic acids, and these metabolites can be used to extract uranium from ores for organic acids can form complexes with uranium. And mixed organic acids produced by fungi are cheaper than those pure organic acids purchased from the market. Mishra et al. [9] isolated Cladosporium oxysporum, Aspergillus flavus and Curvularia clavata, and used them for uranium leaching from a uranium ore with the pulp density of 10 % (w/v), obtaining 71, 59 and 50 % of the uranium recovery, respectively. Hefnawy et al. [10] isolated Aspergillus terreus, Penicillium spinulosum, and used them for uranium leaching form a radioactive geologic sample, obtaining 75 and 81.5 % of the uranium recovery, respectively. Desouky et al. [11] extracted 30 % of uranium from thorium–uranium concentrate with the bioproducts of Aspergillus ficuum.
Compared with A. ferroxidans and other chemoautotrophic bacteria, fungi have four advantages in the leaching of uranium ore [12, 13]. Firstly, chemoorganoheterotrophic fungi can use utilize organic carbon source as substrate and can dissolve uranium at high pH. And the secreted organic acids can react with calcium, aluminum, iron, and other elements in gangue to form complexes with higher solubility. Secondly, in addition to acidic ore, fungi can transform uranium oxides, carbonates, and phosphates to form uranium complexes with carboxylic acids. Thirdly, fungi are the heterotrophic microorganisms characterized by rapid growth rate, large biomass and short extraction cycle [9–11]. For bagasse, mango bark, coconut shells, rice bran, and other industrial and agricultural wastes can be utilized by fungi, both fermentation cost and environment pollution can be greatly reduced. Fourthly, fungal leaching has low anti-corrosion requirement for equipment, since the organic acids produced by fungi are mainly weak acids, which show less environmental hazards than H2SO4 and other strong acids and can be degraded by environmental microorganisms. As an environment-friendly method, fungal leaching is of huge development potential and wide application prospect and has been extensively studied [14, 15].
Over the past years, only the orbital shaker experiments other than the column experiments on fungal leaching of uranium ore have been carried out. But, the column experiments on fungal leaching of uranium ore are indispensable for verifying the feasibility of fungal leaching of uranium ore.
In the present study, an isolated and purified strain of Aspergillus niger with high production capability of organic acids was cultured and its metabolic products were obtained. The column experiment on fungal leaching of uranium ore was carried out with the metabolic products as the leaching agent. Thereafter, the ion exchange experiment was carried out with the leach solution, and the recovery characteristics of complexes formed by uranium and organic acids were discussed.
Materials and methods
Uranium ore
Uranium ore used in the experiment is core samples from a uranium deposit in West China, and the main mineral is pitchblende. The core samples were crushed and then mixed to obtain comprehensive samples. After further splitting, crushing and passing through a 10-mm sieve, ore samples with the particle size of less than 10 mm were obtained and then used for chemical composition analysis. The obtained samples were analyzed by the atomic absorption spectroscopy and the titration, and they were found to have high contents of SiO2 and Al2O3. The content of U6+ and U4+ in the uranium ore was analyzed followed the method of Shen ZQ [16]. The results showed the content of U6+ was significantly higher than that of U4+, and the U6+/U4+ ratio was 4.9 (Table 1). The ore belongs to the easily leached uranium ore.
Strains and media
Aspergillus niger was enriched, separated, and purified in our laboratory. The purified strain was identified with physiological and biochemical methods as well as 26S rDNA analysis by Microbiological Analysis Identification Center in Guangdong Province, China. And the accession number of partial sequence of 26S rDNA of the strain of A. niger is KF990490. The culture medium was the potato sucrose agar (PSA).
Preparation of the metabolic products of Aspergillus niger
Spore suspension of A. niger was prepared and adjusted to the OD600 (optical density of a sample measured at a wavelength of 600 nm) value of 0.1. According to the inoculation amount of 0.1 %, spore suspension was inoculated in PSA and cultured in a constant-temperature (25 °C) shaker at the rotation speed of 200 r/min. When the pH value of the culture solution reached 2.0–2.5, the culture solution was filtered with gauze to remove mycelia. The obtained filtrate, which was the metabolic products of A. niger, was analyzed using high performance liquid chromatography (HPLC), and the analysis results indicated that the metabolic products mainly included oxalic acid, citric acid, and other organic acids. The concentrations of various organic acids detected in the metabolic products were shown in Table 2. The metabolic products were used for column experiments of uranium ore.
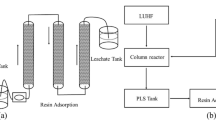
Column leaching and ion exchange
The two columns for leaching were 1,000 mm in height and 100 mm in internal diameter. And the two columns for ion exchange were 1,000 mm in height and 5 mm in internal diameter, and 50 mL of 408 (II) strong base anion exchange resin was loaded into the columns for ion exchange. All the columns were made of plexiglass.
Uniformly mixed uranium ore (10 kg) with the particle size of less than 10 mm was loaded into the column in segments, and the height of the loaded ore was measured to be 900 mm. The drip irrigation method was used. The daily irrigation volume of the metabolic products of A. niger was 1 L. Irrigation time of leaching agent was 12 h. The irrigation intensity was 10.62 L m−2 h−1. The leaching was carried out at room temperature (30–37 °C). The leach solution was collected to determine volume, uranium concentration, Eh value, and pH value. When the uranium concentration in the leach solution was below 50 mg L−1, the leaching was completed and the leached residue was collected to determine the content of uranium. For the control, 7 g L−1 H2SO4 was used for the leaching of the same uranium ore under the same conditions.
The leach solution was subjected to vacuum filtration, and then it was used as the raw liquid for ion exchange. Retention time was controlled to 10 min through regulating the flow rate. The ion exchange effluent was collected in 50 mL (1 bed volume) fractions for analysis.
After the ion exchange was completed, 250 mL water was used for the backwash of the ion exchange column. Then the eluant (1 mol L−1 NaCl and 0.05 mol L−1 of H2SO4) was used for elution. Retention time was controlled to 10 min. The eluate was collected in 50 mL (1 bed volume) fractions for analysis.
Analysis method of uranium
After the added perchloric acid digested the organic material in the leached residue samples to avoid interference, the uranium contents in the leached residue samples were determined by ICP-MS. ICP-MS was also used to determine the uranium concentration in the elution solution.
Results and discussion
Column leaching test
The metabolic products of A. niger and H2SO4 showed the similar leaching cycle and uranium recovery (Table 3). During the leaching process with the metabolic products of A. niger, the pH value was between 2.77 and 3.54 and the Eh value was gradually decreased from 350 to 132 mV. During the leaching process with H2SO4, the pH value of the leach solution declined rapidly and was then maintained at 1.2 and the Eh value was gradually increased from 303 to 448 mV (Figs. 1, 2). In the leach solutions obtained with the metabolic products of A. niger and H2SO4, average uranium concentrations were 689.12 and 660.01 mg L−1, respectively. And the uranium recoveries were 81.76 and 83.90 %, respectively. The results indicated that the leaching process with the metabolic products of A. niger was mild, effective, rapid, and environment-friendly. The uranium recovery from the leach solution obtained with H2SO4 was slightly higher than that from the leach solution obtained with the metabolic products of A. niger. The difference might be interpreted as follows: the majority of iron element was in the ferric state when the oxidation potential was over 400 mV, which was more favorable for uranium leaching [17].
On the first day, the uranium minerals on the surface of the ores were dissolved quickly by the metabolic products of A. niger and H2SO4, and the uranium concentration in the leach solution increased rapidly to its maximum. Compared with H2SO4, the metabolic products of A. niger allowed the higher uranium concentration in the leach solution in this stage, because organic acids could react with U6+ and U4+ to form soluble chelate complexes simultaneously [18, 19], while H2SO4 could only react with U6+ to form soluble complexes [5].
When the uranium minerals on the surface of the ores were almost dissolved, the leaching agents could be accessible to the uranium minerals left through the micro fissures and micro pores within ore particles. In this stage, both of the metabolic products of A. niger and H2SO4 diffused slowly into the inner parts of the ore particles via the micro fissures and micro pores and then reacted with the accessible uranium minerals within the ore particles [20]. As a result, the uranium concentration in the leach solutions were maintained at the relatively low levels from the 2nd to 4th day. However, the uranium concentration in the leach solution from leaching of the uranium ore by the metabolic products of A. niger was lower than that from leaching of the uranium ore by H2SO4 in this stage. (Fig. 3). The difference might be interpreted as follows: firstly, the metabolic products of A. niger were defused into the ore particles more slowly than H2SO4 because the molecular weight of the former was bigger than that of the latter; secondly, slightly insoluble U4+ ions were oxidized into soluble U6+ ions in the H2SO4 leaching system when the oxidation potential increased in the system. In addition, after the pH decreased in the H2SO4 leaching system, the solubility of uranium increased and hydrolysis precipitation of U6+ was prevented.
When the uranium concentrations in the leach solutions from the two leaching systems reached 300 mg/L, they showed the similar variation, and this was probably due to the decrease of the U6+ in the H2SO4 leaching system.
Ion exchange test
The variation of the uranium concentration in the ion exchange effluent from the metabolic products of A. niger leaching system showed a wavy curve. While the variation of uranium concentration in the ion exchange effluent from the H2SO4 leaching system exhibited the S-shaped curve, whose breakthrough was occured at the 85th bed volume. The uranium concentration in the ion exchange effluent reached 502.26 mg L−1 in the 5th bed volume, amounted to the saturation peak in the 15th bed volume, declined rapidly to the minimum value (59.09 mg L−1) in the 45th bed volume, and increased to the saturation peak again in the 105th bed volume (Fig. 4). The variation might be interpreted in the following aspects. In the initial ion exchange stage, stable organic acid-uranium complexes could not be formed on the resin due to the anion interference. Then, anions were eluted from the column and the uranium concentration increased gradually. After all the anions on the resin were substituted by organic acids, uranium was fixed on the ion exchange resin, and the uranium concentration in the ion exchange effluent declined rapidly. Because of the continuous damage caused by organic acids, the resin poisoning occurred and the color of the resin gradually turned black from the top to the bottom of the column. The uranium concentration in the ion exchange effluent rose gradually until the saturation peak was reached again.
Elution experiment
As shown in Fig. 5, the majority of uranium was eluted with a few bed volumes of eluant. The uranium concentrations in the eluates from the two leaching systems reached their peaks (6.65 and 7.29 g L−1) in the 5th and 6th bed volumes, respectively. The H2SO4 leaching system showed the higher peak. The uranium concentration in the eluate was zero in the 20th bed volume. During the elution process, the resin was gradually restored to its original color from the black, indicating that the eluant could alleviate or eliminate the resin poisoning caused by organic acids.
Experiment results of column leaching of uranium ore with the metabolic products of A. niger and H2SO4 showed the similar uranium extraction rate, indicating that fungal leaching of uranium ore was a promising uranium leaching method. However, there were two problems to be solved for this method. Firstly, the recovery of uranium from the leach solution was a problem, because organic acids would rapidly led to resin poisoning, which would resulted in the rapid decrease of ion exchange efficiency. Secondly, the metabolic products of A. niger contained residual spores and nutrients, the residual spores were germinated to form mycelia at the leaching temperature (30–37 °C), and mycelia plugged the elution outlet in the late stage of leaching. Therefore, the above two problems need to be resolved in the application study of column leaching of uranium ore with the metabolic products of A. niger.
Conclusions
-
(1)
Column leaching of uranium ore with the metabolic products of A. niger is a promising and environment-friendly method for exploitation of low grade uranium ores.
-
(2)
The leach solutions obtained with the metabolic products of A. niger and H2SO4 have the different ion exchange characteristics and the similar elution curves. The 408 (II) strong base anion exchange resin is not applicable for the ion exchange of the organic acid-uranium complexes.
References
Maisa MA, Ibrahim EE, Mohamed GE, Abdel SMS, Mona ST, Nilly AK (2014) Effect of mineral constituents in the bioleaching of uranium from uraniferous sedimentary rock samples, Southwestern Sinai, Egypt. J Environ Radioact 134:76–82
Debora MO, Luis GSS, Gregory JO, Susan BO (2014) Acid leaching of a copper ore by sulphur-oxidizing microorganisms. Hydrometallurgy 147–148:223–227
Feng SS, Yang HL, Zhan X, Wang W (2014) Novel integration strategy for enhancing chalcopyrite bioleaching by Acidithiobacillus sp. in a 7-L fermenter. Bioresour Technol 161:371–378
Sina G, Zohreh B, Hadi A, Marzie M, Ata A (2014) Bioleaching of high grade Zn–Pb bearing ore by mixed moderate thermophilic microorganisms. Sep Purif Technol 136:241–249
du Preez JGH (1989) A Review of the industrial processes involving uranium-from the ore to the reactor. Radiat Prot Dosim 26:7–13
Fazzini RAB, Levican G, Parada P (2011) Acidithiobacillus thiooxidans secretome containing a newly described lipoprotein Licanantase enhances chalcopyrite bioleaching rate. Appl Microbiol Biotechnol 89:771–780
Ilyas S, Chi RA, Bhatti HN, Bhatti IA, Ghauri MA (2012) Column bioleaching of low grade mining ores containing high level of smithsonite, talc, sphaerocobaltite and azurite. Bioprocess Biosyst Eng 35:433–440
Ding DX, Liu YL, Li GY, Hu N, Wang YD (2012) Two stage column leaching of uranium from uraninite ore. Adv Sci Lett 5:96–100
Mishra A, Pradhan N, Kar RN, Sukla LB, Mishra BK (2009) Microbial recovery of uranium using native fungal strains. Hydrometallurgy 95:175–177
Hefnawy MA, El-said M, Hussein M, Amin MA (2002) Fungal leaching of uranium from its geological ores in Alloga Area, West Central Sinai, Egypt. J Biol Sci 2:346–350
Desouky OA, El-Mougith AA, Hassanien WA, Awadalla GS, Hussien SS (2011) Extraction of some strategic elements from thorium-uranium concentrate using bioproducts of Aspergillus ficuum and Pseudomonas aeruginosa. Arab J Chem. doi:10.1016/j.arabjc.2011.08.010
Saqib M, Claire SJ, David LP, Keith AM, Ping L, Karen SG (2013) Fungi outcompete bacteria under increased uranium concentration in culture media. J Environ Radioact 120:39–44
Mulligan CN, Kamali M, Gibbs BF (2004) Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J Hazard Mater 110:77–84
Ilyas S, Chi RA, Lee JC, Bhatti HN (2012) One step metal bioleaching of sulphide ore with low concentration of arsenic by Aspergillus Niger and Taguchi orthogonal array optimization. Chin J Chem Eng 20:923–929
Ilyas S, Sarwar S, Bhatti HN (2012) Effect of organic acids produced by Penicillium notatum on the extraction of metals ions from Brown Shale. J Chem Soc Pakistan 34:1040–1047
Shen ZQ, Zheng YF, Li QZ, Zhong ML, Gu DX (1996) Study on analytical method of uranium (IV) and uranium (VI) in uranium ores and uranium-bearing rocks. Uranium Geol 12:48–56
International Atomic Energy Agency (1990) IAEA’s Division of Nuclear Fuel Cycle and Waste Managment. Technical reports series No. 313: Manual on laboratory testing for uranium ore processing, first ed. International Atomic Energy Agency, Vienna
Li WC, Victor DM, Chakrabarti CL (1980) Effect of pH and uranium concentration on interaction of uranium(VI) and uranium(IV) with organic ligands in aqueous solutions. Anal Chem 52:520–523
Suzuki Y, Tanaka K, Kozai N, Ohnuki T (2010) Effects of citrate, NTA, and EDTA on the reduction of U(VI) by Shewanella putrefaciens. Geomicrobiol J 27:245–250
Yin SH, Wu AX, Hu KJ, Wang HJ (2011) Solute transportation mechanism of heap leaching and its influencing factors. J Cent South Univ Technol 42:1092–1098
Acknowledgments
This work was supported by Hunan Provincial Natural Science Foundation of China (Grant Nos. 10JJ2033, 13JJ3077), Scientific Research Fund of Hunan Provincial Education Department (Grant No. 13A083) and Science and Technology Project in Hengyang (Grant No. 2013KJ21).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, YD., Li, GY., Ding, DX. et al. Column leaching of uranium ore with fungal metabolic products and uranium recovery by ion exchange. J Radioanal Nucl Chem 304, 1139–1144 (2015). https://doi.org/10.1007/s10967-015-3957-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-3957-0