Abstract
99mTc-paclitaxel was synthesized by using sodium borohydride as a reducing agent. Greater than 95 % labelling efficiency was achieved. Radiochemical purity of the synthesized 99mTc-paclitaxel was validated by thin layer chromatography (TLC) scanner and high performance liquid chromatography (HPLC). 99mTc-paclitaxel passed in vitro stability tests. Biodistribution and scintigraphy studies were performed in Sprague–Dawley rats. The biodistribution study results of 99mTc-paclitaxel were related mainly to the metabolism and excretion routes followed by the parental drug, paclitaxel. Apart from that, biodistribution of 99mTc-paclitaxel was altered after pre-treatment with cold paclitaxel. Hence, 99mTc-paclitaxel may be used as a tracer for paclitaxel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the use of organometallic radioactive biomolecules for diagnostic and therapeutic purpose has made a tremendous progress. In conjugation with that molecular imaging has achieved a pivotal role in the drug development process. It can provide an essential link between in vitro studies and those performed in vivo [1]. Currently the molecular imaging techniques that employ radiotracers are one of the most sensitive techniques. For drug development studies, radionuclide imaging has received the most attention. Radionuclide imaging can be performed with single photon emission computed tomography (SPECT) or positron emission tomography (PET). SPECT and PET are based on the molecular tracer principle and they are established tools in non-invasive imaging of radiolabelled drugs administered in the nano or picomolar range [2–4]. Drugs administrated at these low amounts do not produce any pharmacological effects which reduce the risk of serious adverse effects in human volunteers or patients. Depending on the ligands and radionuclides used, it is possible to generate pharmacokinetic data of radiolabelled drug molecules in humans using a microdose imaging approach [4]. In this report, paclitaxel was used as a model drug, and 99mTc was used as a γ-emitting radionuclide.
Paclitaxel (C47H51NO14) is a pseudoalkaloid. It acts as an antineoplastic agent due to its inhibitory effect of cellular growth by stabilising the microtubule assembly and, thus, blocking the cell replication in the late G2 mitotic phase of the cell cycle [5]. Paclitaxel is prescribed mainly to treat breast and ovarian cancers, but it is known that various cancer cells can be killed effectively by this drug [6]. A number of clinical trials are going on with paclitaxel to explore its potential for the treatment of various cancers [7]. If we can determine the biodistribution of paclitaxel at microdose level, it will be helpful to monitor drug targets as well as possible reaction of taking new medications in heterogeneous patient populations.
There are different methods to determine biodistribution of drugs such as fluorescence imaging, radiotracing and mass spectrometry. Among these methods, radiolabelling is one of the most suitable methods to determine quantitative biodistribution [8]. To determine biodistribution of drugs, radionuclide 99mTc is used extensively in nuclear medicine for economic reasons, as well as favourable imaging characteristics of technetium (γ energy of 140 keV and 6 h half-life). Stannous salts are used for reduction of sodium pertechnetate (Na99mTcO4) to obtain lower valency state of 99mTc but during labelling by this method radiocolloids are generated [9, 10]. The biodistribution of desired molecule is affected by these radiocolloids. We have to optimize the amount of stannous salts and pH of the reaction for getting maximum labelling efficiency. Some time we have to pass the mixture of compounds through a column to obtain radiocolloids free labelled compound [11]. These processes are time consuming.
In this article, we report a novel, rapid and effective method for radiolabelling of paclitaxel by 99mTc using sodium borohydride as a reducing agent. Sodium borohydride was used for reduction of 99mTc (VII) ions, and subsequent complexation was done with paclitaxel molecules. Synthesized 99mTc-paclitaxel has been undergone for quality control, characterization, biodistribution and scintigraphy studies.
Materials and methods
Paclitaxel was obtained as a gift sample from Fresenius Kabi Oncology Ltd., Kalyani, India. Diethylenetriaminepentaacetic acid (DTPA), and Cremophor EL were purchased from Sigma-Aldrich, St. Louis, USA. Sodium borohydride was purchased from Merck, Hohenbrunn, Germany. 99Mo/99mTc kits were obtained from Board of Radiation and Isotope Technology (BRIT, Mumbai, India). 99mTc extraction was performed in Council of Scientific and Industrial Research-Indian Institute of Chemical Biology (CSIR-IICB), Kolkata, India. All other reagents and solvents were obtained from Merck, Hohenbrunn, Germany or SRL, Mumbai, India, and they are either HPLC or analytical grade. All chemicals were used without further purification. Animal experiments were performed in compliance with the regulations of Institutional Animal Ethics Committee, CSIR-IICB, Kolkata, India. In-house Sprague–Dawley rats (weighing approximately 200–250 g) were used for quantitative biodistribution as well as scintigraphy studies.
Synthesis of 99mTc-paclitaxel
Radiolabelling of paclitaxel was done with previously reported method with modifications [12, 13]. Nitrogen purging, prior to mixing was carried out to degas all solutions. To 100 µl of 99mTc (111 MBq) in saline, 5 mg of solid sodium borohydride was added directly with continuous stirring followed by immediate addition of 500 µl of the paclitaxel solution (1 mg/ml, paclitaxel was dissolved in a mixture of ethanol and Tris–HCl buffer, pH 7.4; ethanol: Tris–HCl buffer = 2:1). The solution was stirred for 45 min at room temperature (25 ± 2 °C). The contents were filtered using 0.22-micron filter (Millipore Corporation, Carrigtwohill, Ireland) and transferred into an evacuated sterile sealed vial and used for further experiment.
Quality control and stability study
Quality control was performed by following the method described earlier [14]. The labelling efficiency of 99mTc to paclitaxel was assessed by ascending instant thin layer chromatography using silica gel plates (ITLC-SG). The ITLC-SG was performed using acetone as the mobile phase. Approximately, 2–3 µl of the radiolabelled complex was applied at the bottom point, 1.0 cm from the end of an ITLC strip. The strip was developed until solvent front reached 8.5 cm from the origin. The labelling efficiency was estimated after dividing the ITLC sheets into two equal halves and counting radioactivity of each segment using gamma ray spectrometer: GRS 23C, ECIL, Mumbai, India. The stability of 99mTc-paclitaxel was checked for 24 h at room temperature. Labelling efficiency was calculated using the following equation.
-
(i)
Labelling Efficiency (%) = [(Total counts − counts of free pertechnetate)/Total counts] × 100 %
Radiochemical purity of 99mTc-paclitaxel and Na99mTcO4
One to two µl of synthesized 99mTc-paclitaxel was applied at the bottom point, 1.0 cm from the end of an ITLC strip (6 cm long). The chromatogram was developed in acetone solution. After the run, the strips were dried and scanned under a mini Gita TLC scanner (Ray test, Straubenhardt, Germany). Similarly, an ITLC of Na99mTcO4 used for the synthesis of 99mTc-paclitaxel was developed in acetone and scanned under the mini Gita TLC scanner.
HPLC analysis of 99mTc-paclitaxel
For HPLC analysis of 99mTc-paclitaxel, reversed phase C-18 column (3.9 mm × 300 mm, µ bondapak column from Waters, Bangalore, India) suitable for radiolabelled molecule detection was used. The mobile phase used in RP-HPLC for gradient system (Gradient I) consisted of water (solvent A) and acetonitrile (solvent B). Gradient I: 0 min 100 % A (0 % B), 2 min 90 % A (10 % B), 6 min 80 % A (20 % B), 12 min 50 % A (50 % B), 25 min 10 % A (90 % B), 30 min 0 % A (100 % B), 35 min 100 % A (0 % B). To obtain chromatogram, 10 µl of the complex was injected into the HPLC system fitted with radioactive detector. Here, flow rate was 1 ml/min.
In vitro stability tests
In vitro stability of the 99mTc-paclitaxel complex was determined in phosphate buffer saline (PBS) [pH 7.4] and in rat serum separately at different time points, at room temperature [15]. The labelled complex (0.1 ml) was incubated with 0.4 ml of PBS or freshly collected rat serum. The stability tests were performed by determining the changes in labelling efficiency. The samples were analyzed by using ITLC at regular intervals up to 24 h. Chromatograms were obtained in gamma ray spectrometer.
DTPA challenge test
In order to check the strength of binding of 99mTc with paclitaxel, 0.1 ml of the labelled preparation was challenged against various concentrations (10, 30 and 50 mM) of DTPA and incubated for 1 h at 37 °C [16]. The effect of DTPA on labelling efficiency was measured on ITLC-SG using acetone as the mobile phase, which allowed the separation of free pertechnetate (R f = 0.9–1.0) and DTPA-complex (R f = 0.8–0.9) from the 99mTc-paclitaxel, which remained at the point of application (R f = 0). ITLC was carried out in 11 cm long ITLC strip. R f values of free pertechnetate and 99mTc-DTPA were validated by using standard solutions of them, respectively. Standard 99mTc-DTPA was prepared as described previously [17], and standard pertechnetate was prepared by adding saline in extracted 99mTc. Percentage Transchelation was calculated by the following formula.
-
(ii)
% Transchelation = [decrease in labelling efficiency of 99mTc-paclitaxel after 1 h incubation with DTPA/labelling efficiency of 99mTc-paclitaxel at 1 h in room temperature] × 100 %
Lipophilicity test of 99mTc-paclitaxel
The octanol/buffer partition coefficient was measured using a standard protocol [18]. Two ml of 1-octanol and two ml of PBS (pH 7.4) were mixed in a centrifuge tube. One hundred µl of 99mTc-paclitaxel was added to the system, and the mixture was vortexed at room temperature for 1 min and then centrifuged at 5,000 rpm (1,398×g) for 5 min. From each phase, 0.1 ml of the aliquot was pipetted and counted in gamma spectrometer. Care was taken to avoid cross contamination between the two phases. The measurement was done in triplicate. The partition coefficient was calculated by using the following equation.
-
(iii)
Log P = Log [(cpm in octanol − cpm in background)/(cpm in buffer − cpm in background)]
Blood clearance and total plasma protein binding study
Blood clearance and plasma protein binding [10] of 99mTc-paclitaxel was studied in rats (Sprague–Dawley) injecting 18.5 MBq (500 µCi) of the radiopharmaceutical through the femoral vein. Blood (250 µl) was collected by puncturing heart of rat. The radioactivity of the withdrawn blood at different time intervals (5 min, 30 min, 1 h, 2 h, 6 h and 24 h) was measured by using gamma ray spectrometer to obtain the blood clearance curve of the complex.
The total plasma protein binding of 99mTc-paclitaxel was determined by 10 % trichloroacetic acid (TCA) method from heparinized blood. Blood cells and plasma were separated by precipitation at 10,000 rpm for 6 min. Plasma proteins were precipitated and isolated by centrifugation for 6 min at 10,000 rpm following the addition of an equal volume of 10 % TCA in the plasma. The supernatant was decanted, and the pellet was re-suspended in 1 ml TCA (10 % v/v). Again centrifugation was done, and the supernatant was decanted. Radioactivity in both the supernatants was measured. This count was expressed as a percentage of count obtained with the same volume of unprocessed plasma.
-
(iv)
% Protein bound = [(counts in plasma − counts in both the supernatants)/counts in plasma] × 100
Quantitative biodistribution study
Biodistribution studies were performed in rats (Sprague–Dawley rats weighing approximately 200–250 g) [13]. 99mTc-paclitaxel complex solution (approximately 500 µCi, 200 µl) was administered through the femoral vein of anaesthetized rats with 0.5 mm polyethylene (PE) catheter. At different time intervals (5 min, 30 min, 1 h, 2 h, 6 h and 24 h), the animals were sacrificed by intravenous injection of air. Urine samples were obtained by puncturing urinary bladder. Other organs (brain, heart, liver, lungs, spleen, muscle, kidney, intestines, stomach and pancreas) were removed, rinsed with saline and blotted to remove any adherent material. The organs were collected in pre-weighed scintillation tubes after drying. Then organs were weighed and radioactivity was counted in a well-type counter (gamma ray spectrometer) with respect to suitably diluted aliquots of the injected solution as standard. The biodistribution of 99mTc labelled paclitaxel in each organ was calculated as a percentage of the injected dose per gram of the tissue (%ID/g).
-
(v)
%ID/g of Tissue = [(counts in sample)/(weight of sample X total counts injected after decay correction)] × 100 %
Quantitative biodistribution study after pre-treatment with cold paclitaxel
This study was done according to previously reported method [19] with modifications. Briefly, 20 mg/kg of paclitaxel was injected intravenously into the Sprague–Dawley rats weighing approximately 200–250 g at day 1. After that, 99mTc-paclitaxel was injected in those rats at day 5 (approximately 500 µCi, 200 µl), and biodistribution of 99mTc-paclitaxel was determined at 30 min, 1 h, 2 h, 6 h and 24 h by following the method indicated in “Quantitative biodistribution study” section.
99mTc-paclitaxel scintigraphy
Imaging studies were performed on normal Sprague–Dawley rat, 200–250 g at Thakurpukur Cancer Research Centre (Regional Radiation Monitoring Centre, Kolkata, India) under dual-head gamma camera (GE Hawkeyes, Pittsburgh, USA). The urethane-anaesthetised rat was injected via the femoral vein with a bolus containing 99mTc-paclitaxel (approximately 500 µCi, 200 µl). Rat was placed in a typical position for planar imaging under a small field of view experimental gamma camera, suitable for both planar and tomographic imaging. Whole body image acquisition study was done at 5 and 90 min after injection, and scan time was 1 min. Image data were obtained and analyzed using a gamma camera (GE Hawkeyes) fitted with a low energy high-resolution all purpose collimator using static procedure of the Xeleris (Functional Imaging) Workstation system.
Statistical analysis
The calculations of mean and standard deviation (SD) were performed on Microsoft Excel. Statistical significance was considered when p < 0.05 and calculations were done using GraphPad InStat (GraphPad Software Inc., San Diego, USA) Software.
Results
More than 95 % of 99mTc was found to label the paclitaxel molecules as calculated from the labelling efficiency equation. The complexation of 99mTc with paclitaxel was found to be rapid, and within 30 min greater than 85 % labelling efficiency was achieved, while labelling efficiency was maximum after 40–45 min (~98 %) (Fig. 1). The resulting complex of 99mTc-paclitaxel was found to be quite stable up to 4 h (labelling efficiency was maintained ~95 %). Then labelling efficiency decreased slightly at 6 h (~94 %), and the calculated labelling efficiency was found greater than 90 % at 24 h (Table 1).
Radiochemical purity of the complex (99mTc-paclitaxel) was checked by TLC scanner. TLC scanning of 99mTc-paclitaxel illustrates that the radiochemical purity of the complex was >95 % (Fig. 2), forming a single major peak at the point of spotting (R f = 0). Similarly, TLC scanning of Na99mTcO4 indicates that the pertechnetate (R f = 0.9–1.0) used for the synthesis of 99mTc-paclitaxel was highly pure (Fig. 2). Synthesized 99mTc-paclitaxel was used without further purification.
Radiochemical purity of the complex (99mTc-paclitaxel) was also validated by RP-HPLC. HPLC analysis of 99mTc-paclitaxel illustrates that the radiochemical purity of the complex was >95 % (Fig. 3), forming a major peak at 8.42 min (retention time of 99mTc-paclitaxel) with minor impurity (i.e. Na99mTcO4, retention time ~ 4.5 min).
In vitro serum stability determination of the radiolabelled complexes is a decisive parameter for their stability. The serum contains proteins which may chelate and bind to 99mTc, disturbing the stability of labelled complexes. Apart from that, physiological pH (i.e. 7.4) may also affect the stability of the complex. It was found that 99mTc-paclitaxel complex was stable in PBS and rat serum (Table 2) for 24 h post labelling. The stability of the labelled complex in PBS and serum supports its stability in biological environment upon administration into the body.
DTPA challenge was performed to get information on the transchelation (a measure of strength of binding) of the synthesized 99mTc-paclitaxel. Transchelation can be defined as a form of chelation in which one chelate group replaces another. When 99mTc-paclitaxel was incubated with DTPA, a small amount of DTPA complex was formed. The formed complex was 99mTc-DTPA as suggested by our TLC studies using standard 99mTc-DTPA. So, transchelation occurred as some amount of 99mTc-paclitaxel was replaced by 99mTc-DTPA. Challenge studies demonstrated that the labelling efficiency of 99mTc-paclitaxel did not alter much in the presence of DTPA (Table 3). At 50 mM concentration of DTPA, the transchelation was found to be 4.76 % for 99mTc-paclitaxel which confirms the in vitro stability of the radiolabelled complex [20].
Log P was calculated from the octanol/buffer (pH 7.4) partition coefficient method. The partition coefficient of the 99mTc-paclitaxel complex was –1.46 ± 0.03 (n = 3). It clearly indicates that the complex was hydrophilic in nature.
The blood clearance curve of 99mTc-paclitaxel (Fig. 4) showed that the initial accumulation of radioactivity in the blood of animal increased up to 1 h, then decreased rapidly in radioactivity up to 6 h. The complex showed a decrease in the clearance rate after 6 h. This indicates a triphasic clearance pattern of the complex from the system. Approximately, 75 % of the complex was removed from the blood after 6 h.
The extent of free 99mTc-paclitaxel in rat plasma was calculated by trichloroacetic acid (TCA) method. TCA method provided the total plasma protein binding level of the complex. The percentage of protein bound drug was found to be 26.13 ± 2.59 (n = 3), indicating that significant amount of the drug was free in rat plasma.
The organ distribution pattern of 99mTc-paclitaxel expressed as the %ID/g of tissue in rats after 5 min, 30 min, 1 h, 2 h, 6 h and 24 h is presented in Table 4. 99mTc-paclitaxel was distributed rapidly after intravenous administration as shown by its biodistribution. Although intestine, stomach, kidney, urine and urinary bladder uptake were noteworthy, but liver uptake of the complex was maintained a high level up to 2 h of biodistribution study (at 5 min: highest uptake; at 30 min, 1 h and 2 h: 3rd highest uptake; Table 4). It is known that paclitaxel is mainly metabolized in the liver. The organ distribution pattern of 99mTc-paclitaxel indicates that the same in vivo system (i.e. liver) is responsible for the metabolism of 99mTc-paclitaxel. Biodistribution of 99mTc-paclitaxel also suggests that the complex was rapidly eliminated mainly through hepatobiliary system and partially through kidney (Table 4).
The biodistribution (%ID/g) of 99mTc-paclitaxel after pre-treatment with cold paclitaxel is presented in Table 5. Here, it was observed that the relative organ distribution pattern of 99mTc-paclitaxel was similar to 99mTc-paclitaxel injected alone, but with decreased %ID/g (p < 0.05 for most of the data) of tissue. It was in agreement with the previously reported literature where it was shown that paclitaxel pharmacokinetics is altered by previous paclitaxel exposure up to 96 h earlier [19]. These data suggest that 99mTc-paclitaxel can be a tracer for paclitaxel in rats.
Whole body images of normal rats at 5 min and 90 min after 99mTc-paclitaxel administration are presented in Fig. 5. The complex was seen in rat liver soon after intravenous injection, and it was distributed to almost every organ as it was found in biodistribution study. At 90 min, the accumulation of radioactivity has been observed mostly in liver as well as in intestine and stomach. Apart from that distribution of 99mTc-paclitaxel can also be observed in urinary bladder.
Discussion
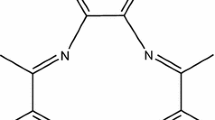
Paclitaxel is practically insoluble in water with extremely low aqueous solubility (less than 0.3 mg/ml) [21]. It is soluble in organic solvents like acetone, ethanol and acetonitrile. Complete IUPAC name of paclitaxel is tax-11-en-9-one-5β, 20-epoxyl-1, 2α,4,7β,13α-hexahydroxy-4,10-diacetate-2-benzoate 13-β-benzoylamino-α-hydroxybenzenepropionate (structural formula shown in Fig. 6). Synthesized 99mTc-paclitaxel was characterized radiochemically. Single peak in TLC scanner confirms the radiochemical purity of the synthesized 99mTc-paclitaxel. It also validates the identity of 99mTc-paclitaxel with respect to Na99mTcO4. HPLC analysis of 99mTc-paclitaxel also supports our observation in ITLC. We found that the complex was fairly stable in room temperature. Paclitaxel possesses four main binding sites (=O, −OH, > NH, and = O at 1′, 2′, 3′ and 4′ position of paclitaxel molecule, respectively; Fig. 6) which can participate to form stable complex with 99mTc. Probably, two molecules of paclitaxel bind with 99mTc (Fig. 7), which can explain the stability of the formed complex. We expected the structure of 99mTc-paclitaxel from literature [22] and MM2 energy minimized structure by energy minimization computation of all possible structures using Chem-3D ultra software (Fig. 8) [23]. It has been reported in the literature that 4, 5 or 6 membered ring containing 99mTc can form stable complex [22].
We have also evaluated 99mTc-paclitaxel biologically. Evaluation of radiolabelled paclitaxel in animals is important to the study of its possible use for the prediction of chemotherapeutic efficacy with paclitaxel in cancer patients. Rapid clearance (as well as clearance pattern) of 99mTc-paclitaxel from the system, its liver accumulation as well as elimination pattern, and organ biodistribution data after pre-treatment with cold paclitaxel suggest that synthesized complex has the potential to be used as a tracer for paclitaxel in vivo. The organ distribution of 99mTc-paclitaxel was related mainly to the metabolism and excretion routes reported for the parental drug, paclitaxel [24]. This study confirms that 99mTc-paclitaxel biodistribution provides a good estimate of paclitaxel biodistribution. Therefore, 99mTc-paclitaxel may be able to predict the uptake of paclitaxel in solid tumors. Apart from that, paclitaxel is a known substrate for P-glycoprotein (Pgp). Pgp is a membrane pump whose overexpression has been shown to result in multidrug resistance (MDR) [25]. For this reason, it may also be assumed that 99mTc-paclitaxel may have role to image MDR, however, comparison with 99mTc-sestamibi and other related MDR imaging radiotracers is mandatory.
99mTc radiopharmaceutical imaging is used in approximately 85 % of nuclear medicine procedures [26], and it is well known that cost effectiveness of SPECT imaging is higher than that of PET imaging. Previously, a number of articles described the radiolabelling of paclitaxel with different radioisotopes [11, 27, 28] for SPECT imaging. 99mTc-paclitaxel was synthesized by stannous chloride method [11], but its potential as a tracer for paclitaxel was not evaluated. Indium-111-DTPA-paclitaxel was synthesized and biologically evaluated [27]. Limitation with indium-111 is that its high affinity for transferrin receptors. This leads to excessive radiation burden to the bone marrow of patients treated with indium-111 labelled product. I123 was also used to radiolabel paclitaxel, but this complex was not biologically evaluated [28].
In this work, we radiolabelled paclitaxel with 99mTc by using sodium borohydride as a reducing agent. Sodium borohydride has long been used in radiolabelling purpose [12, 13, 29]. It is worth noting that radiolabelling by tricarbonyl method also uses sodium borohydride in labelling procedure [30]. Boric acid is generated as a by-product when sodium borohydride is used in radiolabelling purpose [29]. It has been reported in the literature that up to 611 mg of boric acid was administered by the intravenous route in human being with no signs of toxicity [31]. It has also been reported that 350 parts per million (ppm) of boric acid had no adverse effects on fertility, lactation, weight or appearance of rats [32]. The amount of boric acid generated by the reaction described in this work was less than 10 mg, and in agreement with the literature report we haven’t seen loss of weight or change of appearance in Sprague–Dawley rats during 24 h biodistribution study.
Anaphylaxis and severe hypersensitivity reactions characterized by dyspnea and hypotension requiring treatment, angioedema, and generalized urticaria have occurred in some patients receiving paclitaxel (Taxol) in different clinical trials. Fatal reactions occurred in patients despite premedication [33]. Taxol formulation contains paclitaxel dissolved in a 50:50 (v/v) mixture of the surfactant polyoxyethylated castor oil (Cremophor EL) and dehydrated ethanol. Cremophor EL has been shown to cause those acute hypersensitivity reactions, one of the most severe side effects associated with discontinuation of paclitaxel therapy [34]. We radiolabelled paclitaxel in the presence of Cremophor EL (composition similar to Taxol formulation and amount of paclitaxel 1 mg/ml) by the method described in this article. We have found similar radiochemical purity and stability data, but we did observe altered total plasma protein binding (71.54 ± 2.06, n = 3) and biodistribution pattern [13] in the presence of Cremophor EL as indicated by others also [35–37]. It has been demonstrated that Cremophor EL induced the appearance of a lipoprotein dissociation product for which paclitaxel has a high affinity [35], and Cremophor EL can change the biodistribution of drugs [38].
Dose, regimen and effect of combination of paclitaxel with other anticancer drugs are still evolving [7]. Further, low-dose paclitaxel has shown antiangiogenic activity in vivo and this antiangiogenic activity of paclitaxel was not linked with its cytotoxicity [39]. It is worth noting that chemotherapy doses of paclitaxel (minimum dose 180 mg/m2) are quite high compared to 200 µg dose of 99mTc-paclitaxel used for imaging purpose. A maximum activity of 740 MBq (20 mCi) was used to radiolabel 80 µg of paclitaxel and no effect on stability and radiochemical purity was noticed as such when compared to 111 MBq (3 mCi) 99mTc-paclitaxel. Multiple scan of a patient may be performed during the course of chemotherapy by using 20 mCi 99mTc-paclitaxel [18]. Hence 99mTc-paclitaxel may also be used as a tracer for paclitaxel in human being. Possible reaction of taking new medications and treatment modalities can be determined based on the test results obtained from administration of 99mTc-paclitaxel.
Conclusion
Radiosynthesis of 99mTc-paclitaxel was done successfully by using sodium borohydride as a reducing agent. No such interference of colloid was observed by this labelling procedure as both the radiolabelling efficiency (97.79 ± 0.42, n = 3) and radiochemical purity (96.77 ± 0.31, n = 3) of the complex was found to be >95 %. Labelling of paclitaxel by sodium borohydride was found to be easy and effective. The complex was hydrophilic in nature, and the majority of this complex (without Cremophor EL) remains free in plasma. Cremophor EL has a profound effect on biodistribution and plasma protein binding of 99mTc-paclitaxel. Scintigraphy and biodistribution indicate that significant accumulation site for 99mTc-paclitaxel is liver for up to 2 h. The doses of 99mTc-paclitaxel can be reduced by using higher activities of 99mTc in labelling procedure. Pre-treatment with cold paclitaxel altered the biodistribution of 99mTc-paclitaxel in rats. These studies endorse that 99mTc-paclitaxel has the potential to be used as a tracer during the course of chemotherapy, however further evaluation is mandatory.
References
Pomper MG, Lee JS (2005) Small animal imaging in drug development. Curr Pharm Des 11:3247–3272
Franc BL, Acton PD, Mari C, Hasegawa BH (2008) Small-animal SPECT and SPECT/CT: important tools for preclinical investigation. J Nucl Med 49:1651–1663
Golestani R, Wu C, Tio RA, Zeebregts CJ, Petrov AD, Beekman FJ, Dierckx RA, Boersma HH, Slart RH (2010) Small-animal SPECT and SPECT/CT: application in cardiovascular research. Eur J Nucl Med Mol Imaging 37:1766–1777
Gomes CM, Abrunhosa AJ, Ramos P, Pauwels EK (2011) Molecular imaging with SPECT as a tool for drug development. Adv Drug Deliv Rev 63:547–554
Horwitz SB (1992) Mechanism of action of taxol. Trends Pharmacol Sci 13:134–136
Lin HL, Liu TY, Chau GY, Lui WY, Chi CW (2000) Comparison of 2-methoxyestradiol-induced, docetaxel-induced, and paclitaxel-induced apoptosis in hepatoma cells and its correlation with reactive oxygen species. Cancer 89:983–994
http://www.clinicaltrials.gov/. Accessed 31 July 2014
Liu Y, Tseng YC, Huang L (2012) Biodistribution studies of nanoparticles using fluorescence imaging: a qualitative or quantitative method? Pharm Res 29:3273–3277
Behera A, Banerjee I, De K, Munda RN, Chattopadhayay S, Samanta A, Sarkar B, Ganguly S, Misra M (2012) Synthesis, characterization, conformational analysis of a cyclic conjugated octreotate peptide and biological evaluation of (99 m)Tc-HYNIC-His (3)-Octreotate as novel tracer for the imaging of somatostatin receptor-positive tumors. Amino Acids 44:933–946
De K, Bhowmik A, Behera A, Banerjee I, Ghosh MK, Misra M (2012) Synthesis, radiolabeling, and preclinical evaluation of a new octreotide analog for somatostatin receptor-positive tumor scintigraphy. J Pept Sci. doi:10.1002/psc.2458
Jain V, Swarnakar NK, Mishra PR, Verma A, Kaul A, Mishra AK, Jain NK (2012) Paclitaxel loaded PEGylated gleceryl monooleate based nanoparticulate carriers in chemotherapy. Biomaterials 33:7206–7220
Banerjee T, Mitra S, Kumar Singh A, Kumar Sharma R, Maitra A (2002) Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int J Pharm 243:93–105
Banerjee I, De K, Chattopadhyay S, Bandyopadhyay AK, Misra M (2014) An easy and effective method for radiolabelling of solid lipid nanoparticles. J Radioanal Nucl Chem. doi: 10.1007/s10967-014-3258-z
Theobald AE (1990) Textbook of radiopharmacy: theory and practice. Gorden and Breach, New York
Reddy LH, Sharma RK, Murthy RS (2004) Enhanced tumour uptake of doxorubicin loaded poly (butyl cyanoacrylate) nanoparticles in mice bearing Dalton’s lymphoma tumour. J Drug Target 12:443–451
Mishra AK, Iznaga-Escobar N, Figueredo R, Jain VK, Dwarakanath BS, Pérez-Rodríguez R, Sharma RK, Mathew TL (2002) Preparation and comparative evaluation of 99 mTc-labeled 2-Iminothiolane modified antibodies and CITC-DTPA immunoconjugates of anti-EGF-receptor antibodies. Methods Find Exp Clin Pharmacol 24:653–660
http://www.britatom.gov.in/docs/rph_ria_cat/RPH_COLD/RPH_TCK7.pdf. Accessed 17 Oct 2014
Faheem AR, Bokhari TH, Roohi S, Mushtaq A, Sohaib M (2013) 99mTc-Daunorubicin a potential brain imaging and theranostic agent: synthesis, quality control, characterization, biodistribution and scintigraphy. Nucl Med Biol 40:148–152
Gustafson DL, Long ME, Bradshaw EL, Merz AL, Kerzic PJ (2005) P450 induction alters paclitaxel pharmacokinetics and tissue distribution with multiple dosing. Cancer Chemother Pharmacol 56:248–254
Harivardhan Reddy L, Sharma RK, Chuttani K, Mishra AK, Murthy RS (2005) Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J Control Release 105:185–198
Wenk MR, Fahr A, Reszka R, Seelig J (1996) Paclitaxel partitioning into lipid bilayers. J Pharm Sci 85:228–231
Zolle I (2007) Technetium-99 m pharmaceuticals: preparation and quality control in nuclear medicine. Springer, Berlin
De K, Behera A, Banerjee I, Sarkar B, Ganguly S, Misra M (2014) Radiolabeled novel peptide for imaging somatostatin-receptor expressing tumor: synthesis and radiobiological evaluation. J Radioanal Nucl Chem. doi:10.1007/s10967-014-3199-6
Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH (1998) Preclinical pharmacokinetics of paclitaxel and docetaxel. Anticancer Drugs 9:1–17
Kurdziel KA, Kalen JD, Hirsch JI, Wilson JD, Agarwal R, Barrett D, Bear HD, McCumiskey JF (2007) Imaging multidrug resistance with 4-[18F]fluoropaclitaxel. Nucl Med Biol 34:823–831
Eckelman WC (2009) Unparalleled contribution of Technetium-99m to medicine over 5 decades. JACC Cardiovasc Imaging 2:364–368
Li C, Yu DF, Inoue T, Yang DJ, Tansey W, Liu CW, Milas L, Hunter NR, Kim EE, Wallace S (1997) Synthesis, biodistribution and imaging properties of indium-111-DTPA-paclitaxel in mice bearing mammary tumors. J Nucl Med 38:1042–1047
Roh EJ, Park YH, Song CE, Oh SJ, Choe YS, Kim BT, Chi DY, Kim D (2000) Radiolabeling of paclitaxel with electrophilic 123I. Bioorg Med Chem 8:65–68
Banerjee T, Singh AK, Sharma RK, Maitra AN (2005) Labeling efficiency and biodistribution of Technetium-99m labeled nanoparticles: interference by colloidal tin oxide particles. Int J Pharm 289:189–195
Halder KK, Nayak DK, Baishya R, Sarkar BR, Sinha S, Ganguly S, Debnath MC (2011) (99m)Tc-labeling of ciprofloxacin and nitrofuryl thiosemicarbazone using fac-[(99m)Tc(CO)3(H2O)3] core: evaluation of their efficacy as infection imaging agents. Metallomics 3:1041–1048
Jansen JA, Andersen J, Schou JS (1984) Boric acid single dose pharmacokinetics after intravenous administration to man. Arch Toxicol 55:64–67
http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500011109.pdf. Accessed 31 July 2014
http://packageinserts.bms.com/pi/pi_taxol.pdf. Accessed 31 July 2014
Koziara JM, Lockman PR, Allen DD, Mumper RJ (2004) Paclitaxel nanoparticles for the potential treatment of brain tumors. J Control Release 99:259–269
Sykes E, Woodburn K, Decker D, Kessel D (1994) Effects of Cremophor EL on the distribution of taxol to serum lipoproteins. Br J Cancer 70:401–404
Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH (1996) Nonlinear pharmacokinetics of paclitaxel in mice results from the pharmaceutical vehicle Cremophor EL. Cancer Res 56:2112–2115
Scripture CD, Figg WD, Sparreboom A (2005) Paclitaxel chemotherapy: from empiricism to a mechanism-based formulation strategy. Ther Clin Risk Manag 1:107–114
Woodburn K, Chang CK, Lee S, Henderson B, Kessel D (1994) Biodistribution and PDT efficacy of a ketochlorin photosensitizer as a function of the delivery vehicle. Photochem Photobiol 60:154–159
Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, Giavazzi R, Taraboletti G (1996) The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res 2:1843–1849
Acknowledgments
The authors gratefully acknowledge Prof. Siddhartha Roy (Director), CSIR- Indian Institute of Chemical Biology (CSIR-IICB), Kolkata, India for his support. We are also thankful to Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE) and CSIR for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, I., Behera, A., De, K. et al. Synthesis, characterization, biodistribution and scintigraphy of 99mTc-paclitaxel: a potential tracer of paclitaxel. J Radioanal Nucl Chem 304, 633–643 (2015). https://doi.org/10.1007/s10967-014-3825-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3825-3