Abstract
Present work deals with the distribution study of naturally occurring radioactive materials in drinking water samples around Orissa sands complex, Odisha, India and assessment of the comprehensive ingestion dose to members of the public. The mean total effective dose to member of public is found to be 67.1 µSv/y with a range of 13.2–198.5 µSv/y. The average effective dose is below the reference level of 100 µSv/y and also comparable with the range of global annual ingestion dose value reported worldwide. The activity concentration in drinking water reported here is due to natural distribution of radioanuclides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Naturally occurring radioactive materials (NORM) are ubiquitous in the environment. Apart from the terrestrial radionuclides (and their decay products) of primordial origin with half-lives comparable to the age of the Earth (about 3 billion years), NORM include cosmic and cosmogenic radionuclides which consists of primary charged and neutral particles that bombard the Earth’s atmosphere and the secondary particles generated by the primary particles in the Earth’s atmosphere. Natural radionuclides present in every compartment of environment with wide variation in concentration depending on regional geological formation, prevailing environmental conditions etc. NORM in the environment mainly arises due to 238U and 232Th series isotopes and 40K. Natural radiation is the largest contributor to the total radiation dose to population. About 20 % of the natural radiation dose is due to external radiation from terrestrial radioactivity [1]. The levels of terrestrial radiation are related to the soil type and consequently to the parent material (type of rocks) [1]. The radionuclides that are present in soils and host rocks can be leached to the corresponding aquifers depending upon their solubility and geochemistry and hence prevails in surface and ground water bodies. There have been reported studies worldwide regarding the occurrence of natural radioactivity in drinking water from different sources [2–5, 6]. Water intake is an essential part of human diet, may contribute to chronic natural radioactivity exposure. Drinking water consumption may be crucial in the areas prone to exposure of natural alpha emitters [1]. Groundwater quality is severely affected by the inherent bedrock and soil characteristics of the area. The areas with granitic host rock contains inherent high level of natural radioactivity and hence groundwater quality is severely affected by the inherent bedrock (e.g. High level of radioactivity granitic host rocks [7]) and soil characteristics of the area. Radiological characterisation of drinking water, by estimating the concentration of these naturally occurring radioactive elements is pivotal to assess the potential radiological impact and dose to the population inhabited in that area.
Few regions in China, Iran, Brazil and India, etc. however were known as natural high background radiation areas (NHBRA) due to monazite deposits where very high terrestrial radiation dose values were reported which have radiation levels (10–100) times higher than the normal background [1]. Details of other NHBRA areas worldwide were discussed elsewhere [8]. Areas such as Guarapari, the coastal region of Espirito Santo and the Morro Do Forro in Minas Gerais in Brazil [9–13]; Yangjiang, in China [14, 15]; the southwest coast of India [16–19]; Ramsar and Mahallat in Iran [20, 21]; in the United States and Canada [22], and in some other counties [1] are well known for their high background radiation due to the local geology. Like in Brazil, China and coastal lines of India, the monazite placer deposit of these regions plays a host to high background radiation as monazite and zircon in rich in terrestrial radionuclides of thorium and uranium. Ramsar, Iran is among the list due to its high level of radium in soil/water and radon in air which contributes to the high background radiation exposure. In India namely Ullal in Karnataka [23], Kalpakkam [24] in Tamil Nadu, coastal parts of Tamil Nadu and Kerala state and the southwestern coast of India are known high background radiation areas [17, 18]. Inhabitants of HBRAs are subjected to high natural radiation exposure which is inevitable. The major source responsible for this exposure is the primordial radionuclides in earth crust like 238U series and 232Th series and 40K. These HBRAs have been under study for many years in order to determine the risks and effects of long-term, low-level and natural radiation exposure [25]. The natural radioactivity scenario may modify drastically due to uncontrolled anthropogenic activities. Mineral mining is one of the anthropogenic practices which has the potential to redistribute the minerals in the adjoining area and may enhance natural radioactivity if adequate safety measures are not implemented [26, 27]. Extraction of ores and subsequent processing technology may lead to enhanced levels of NORM in the surrounding areas and hence mining activities in that particular area may redefine the radiological scenario and modify the environment. The continuous environmental surveillance has been effective in controlling the environmental release of radioactivity since the commencement of the operation.
India is blessed with vast resources of rare earths in the form of monazite found in the beach sands of Kerala, Tamil Nadu and Odisha coast. Indian Rare Earths Limited (IREL), under the Department of Atomic Energy, has been operational in those areas since decades to produce rare earths and thorium from monazite. In India, the beach placer deposit at Chhatrapur coast of Orissa, Manavalakurichi in Tamil Nadu and Chavara in Kerala known for their enhanced radiogenic environment. Orissa Sands Complex (OSCOM) of Indian Rare Earth Limited is located near Mathikhalo village, in Chhatrapur taluk, Ganjam district, Odisha, India and has a mineral separation plant (MSP) for the production of ilmenite, garnet, silemenite, monazite, rutile, zircon, etc. [28]. The area is extended over 20 km ranging from north–east to south–west with an average width of more than 1.5 km. This region has Bay of Bengal in its southern side and Eastern Ghat Groups of rocks in the northern and north western side. Rushikulya River serves as the main drainage system of this region, which originates from Eastern Ghat and joins the sea at Chhatrapur region. Well developed sand dunes are there in this region, parallel to the sea coast [29]. The average heavy mineral concentration in sands from this region varies between 18–23 vol% [30]. The heavy minerals present in the sand are silemenite, ilmenite, zircon, garnet, rutile and monazite. Other reported values of natural gamma radiation survey and estimation of terrestrial natural radioactivity concentration such as 238U, 232Th and 40K in the study area were discussed in the following studies [8, 29, 31, 32].
Study of the natural radioactivity levels in various environmental matrices is of great radiological significance for establishment of natural radiation and dose rate levels around a beach sand mining facility at Odisha and finding out the main radionuclides of concern posing threat to the general public inhabiting this region. In addition, this aids in planning and development of appropriate monitoring and surveillance programme. Also it will generate a database which will be helpful in establishing a NORM map for the study area. This map will be used as reference information to assess any changes in the radioactivity levels in the course of operation of mining activities. Objective of the present study is to appraise the comprehensive radiation dose to the member of public residing around a NHBRA like Chattrapur, Odisha, India due natural radioanuclides in drinking water intake since NHBRA areas are associated with elevated level of natural radioactivity. The present work deals with measurement of the radionuclide concentrations like total uranium, 226Ra, 210Po and 210Pb in drinking water around the study area and assessment of the comprehensive ingestion dose to members of the public. These are the most significant radionuclides typically found in drinking water with respect to radiation dose evaluation. Though the study area has enhanced level of thorium in natural radioactivity concentration due to its granitic host rock but since thorium is water insoluble we concentrated mainly on uranium and its daughters.
Study area and sampling
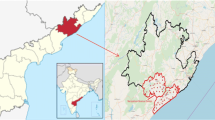
The study area is one of the natural high background radiation area of India, situated around the monazite rich placer deposit in Chatrapur, Ganjam district (19°15′–19°21′N, 84°3′–84°55′E) of Odisha state in eastern India. The high natural background area is along the coast, about 20 km long and 2 km wide. Orissa Sands Complex (OSCOM) is located near Mathikhalo village, in Chatrapur taluk, Ganjam district, Odisha and India Rare Earth Limited (IREL) operates here to recover heavy minerals from the beach sand. The facility consists of a dredging and wet concentrator plant, mineral separation plant (MSP) and a thorium plant commissioned in 1992. The MSP is used for the separation of heavy minerals like sillimanite, monazite, rutile and zircon, etc. The people residing around the area are subjected to higher levels of natural background radiation due to presence of naturally enhanced radionuclide levels in the environment [8].
Location map and zoning of the sampling area is shown in Fig. 1. The surrounding environment up to 10 km around OSCOM was selected for sampling because within this region the impact of the radionuclides will be significant if any. The selected area was divided into three zones with respect to the facility and drinking water samples of one liter each were collected from the surrounding villages of that area following International Atomic Energy Agency (IAEA) recommendation and guideline [33]. Drinking water samples of 24 numbers were collected from both surface and ground water resources (mostly tube well and bore well water) in pre acid cleaned containers in order to avoid wall absorption. Water quality parameters like pH, electrical conductivity (EC) and total dissolved solid (TDS) were measured insitu, using portable handheld instruments. Drinking water samples were filtered using 0.45 µm filter paper for the removal of suspended particulate matter and processed accordingly for the determination of natural radioelement of uranium and 238U progenies of 210Po, 210Pb and 226Ra.
Analytical procedure
Estimation of total uranium
Drinking water samples were directly analysed for total uranium concentration by laser uranium analyser. Laser fluorimetry is one of the quick, sensitive and reliable methods of estimating uranium in ultra trace level in aqueous environmental samples without any sample pretreatment. The fluorescence of uranyl complex was measured in the sample directly after addition of sodium pyrophosphate as fluorescence enhancing as well as complexing reagent. The signal was measured in a photomultiplier tube. The water samples were analyzed by standard addition method, in order to avoid the matrix effect. The minimum detectable activity (MDA) of the method is 0.2 µg/l. The details of the method and analytical procedure are found elsewhere [34, 35]. The recovery of spiked uranium is 70–94 %.
Estimation of 210Po
Generally the activity concentration in water bodies is typically low due to strong binding of polonium with sediment [36]. For the estimation of 210Po in drinking water, samples were first digested with concentrated HNO3 and HCl followed by autodeposition on silver planchette and subsequently counted in alpha spectrometry. Ascorbic acid was added to the solution in order to reduce iron and minimize interference during autodeposition [37]. As the alpha energy is used for detection and measurement, the 210Po requires separation from the bulk matrix and preparation of a thin, uniform monomolecular source for accurate measurement [38]. Auto deposited silver disk samples were counted in 450 mm2 Passivated Ion Implanted Planar Silicon alpha detector (PIPS ULTRA, bias 50 V). 209Po tracer was added to the sample to compute the yield. Results were corrected to the time of sample collection in order to account the 210Po decay. Details about the alpha detector, optimization and counting is given elsewhere [39, 40]. The MDA of the method is 1.5 mBq. The tracer recovery of 209Po is 75–85 %.
Estimation of 210Pb
210Pb was measured by gross beta counting system after radiochemical separation [41]. After the autodeposition of 210Po, the solution was treated with concentrated HNO3 and Pb carrier was added along with H2SO4 in order to get the PbSO4 precipitate. The precipitate was then dissolved in hot saturated solution of ammonium acetate. Na2S solution was added to get PbS precipitate. The precipitate was then dissolved with conc. HNO3 and H2SO4 was added to get the PbSO4 precipitate. The precipitate was then transferred to an Al planchette and after drying, kept for about 35 days to ensure secular equilibrium of 210Pb with its daughter product 210Bi. The planchets were then counted for beta activity by using a silicon detector based simultaneous alpha beta counter (Doza, YMφ-2000) and 210Pb activity was calculated by using the following formula, (Eq. 1), [42].
where A t is the net count rate (s−1), λ Bi is the 210Bi decay constant (min−1), t is the 210Bi in-growth time (min), η is the detection efficiency for 210Bi and w is the sample dry weight (kg). The MDA of the method is 0.2 mBq. The chemical yield of 210Pb is up to 80 %.
Estimation of 226Ra
The activity concentration of radium in ground water mainly depends upon the chemical and physical process like adsorption, desorption, complex formation and precipitation-dissolution which again depends upon the chemical composition of groundwater. The concentration of 226Ra in drinking water was estimated by emanometry technique [43]. Filtered water samples were treated with conc. HNO3 and pre-concentrated prior transferring to the radon bubbler. The radon in the water samples was removed by a vacuum pump and then the water in the bubbler was allowed to stand for 10–20 days in order to build up of fresh radon from the 226Ra present in the sample. The radon gas was then collected in a scintillation cell and counted through a photo multiplier assembly for alpha activity. The 226Ra content of the solution can be derived as follows (Eq. 2) by using the appropriate build up and decay factors for 222Rn, which are a function of the buildup period and decay constant of 222Rn. The MDA of the method is 3.5 mBq.
where D—Net alpha counts obtained (D = c – b, c is the total counts, and b is the background counts), V—Volume of water in radon bubbler (ml), E—Efficiency of the scintillation cell, λ—Decay constant of radon (2.098 × 10−6 s−1), T—Counting delay (s), t—Counting duration (s), Θ—Radon build up period (s).
Quality assurance
The accuracy and reliability of the method is verified by analyzing standard reference material, cross method checking, replicate analysis and spike recovery study. Water samples were analysed for total uranium concentration in laser fluorimetry, alpha spectrometry, and mass spectrometry (ICPMS). The results were in good agreement with each other and the details are given in Table 1. Uranium standard of 5 µg/l is analysed by the above three instrumental techniques in five replicate analysis in order to get the comparison in terms of relative bias and the relative bias associated with the measurement of total uranium by laser fluorimeter, alpha spectrometry and ICPMS were 4, 8 and 14 % respectively. Table 2 depicts the recovery study of 209Po tracer and uranium in certified reference materials. The lower limit of detection (LLD) and the minimum detection activity (MDA) for each method were calculated using either the Currie definition or the formula presented by the Environmnetal Protection Agency (EPA) [44, 45]. The repeatability of all the methods is around 10 %. For 210Po determination, a primary standard solution of activity156.8 ± 1 (±0.8 %) Bq/gm of 209Po solution (Supplied by R33-02 (NPL, National Physical Laboratory, UK) is used for spike recovery study. Secondary standard solution of different activity concentration was prepared by adding appropriate amount of primary standard solution. For determination of uranium in drinking water samples, a certified material (Aldrich) (Atomic Absorption Standard for U determination: activity 1,000 µg/ml) is used for recovery study. The accuracy and precision of the method were evaluated using the supplied certified tracers and standards and the results were in good agreement with the recommended value with a relative error <20 %.
In order to avoid cross contamination, all laboratory glass wares used for sample processing were soaked in 10 % nitric acid for 15 days and then rinsed thoroughly twice with ultra pure water (resistivity 18.1 MΩ, Thermo nanopure diamond TII water purification system) before use.
Results and discussion
Distribution of radionuclides
The value pH, TDS and EC of the water which were measured insitu varied in the range of 6.3–8.3, 0.2–4.5 mS/cm and 191–3,570 µg/ml, respectively. The measured activity concentration of total uranium in drinking water samples by laser fluorimetry was varied between <0.2 and 13.6 ± 1.5 µg/l with an average value of 4.3 µg/l. The mean value of specific activity of uranium was 105.6 ± 7.2 mBq/l with a range of <4.9–334.2 mBq/l. The activity concentration of 226Ra, 210Po and 210Pb ranged between <3.5–39.0 ± 3.5, 1.8 ± 0.1–11.0 ± 2.1 and 3.8 ± 0.6–49.7 ± 5.4 with average values of 15.4, 4.8 and 21.3 mBq/l, respectively. The average uranium concentration (105.6 mBq/l) in ground water is much higher than the average 226Ra concentration which can be attributed to high mobility of 226Ra than uranium. The ratio of measured uranium and 226Ra in drinking water samples vary from 0.1–83.7 with a mean value of 8.0. Thus there was a weak correlation (0.13) observed between the concentration of uranium and 226Ra in water samples analysed, which is also an indicator of different geochemical behavior of uranium and radium. The concentration of NORM in drinking water samples were compared to the results obtained from the drinking water samples collected around Jaduguda, India [46]. The concentration of radium in drinking water is comparable in other reported values of Vizag and Odisha, India [47, 48]. The concentration of uranium in drinking water samples was comparable with the reported value of Vizag, India [49], since Vizag, AP, India has the similar mineralization as the study area [50]. The concentration of uranium and radium obtained in the present study is comparable with the similar study in Jaduguda, India [43, 51]. The concentration of radium and uranium obtained in the present study is comparable with the reported values of Syria [51]. The concentration of NORM value obtained from drinking water analysis in the present study is compared with the other reported data worldwide (Table 3). The concentration of uranium in all the drinking water samples was below the guideline value of 30 µg/l [53, 54] and 60 µg/l [55]. The mean activity concentration of 226Ra was 15.4 mBq/l which is much lower than the WHO guideline value of 1 Bq/l [53]. The activity concentration of 210Po and 210Pb were also lower than the WHO guideline value of 100 mBq/l [53].
Distributions of NORM in drinking water are plotted in Box–Whisker and Histogram plot as shown in Figs. 2 and 3 respectively. From the Box plot it is clear that the data doesn’t follow normal distribution as mean is not equal to median. The wide variation of data is clear from the Box plot. 210Po data deviates from log normal distribution. The statistical parameter of NORM data is listed in Table 4. Large spreading of the 210Pb data is showing in its large variance value. The NORM data are positively skewed means have distributions with an asymmetric tail extending toward more positive values. Negative kurtosis of the data indicates relatively flat distribution.
The contour map of radionuclide concentration distribution is shown in Fig. 4a–d. Contour map was drawn using surfer and the kriging method for griding the data. The IREL industry is located along the coordinates 19.322°N and 84.947°E and indicated in the figure. From the contour maps it is clear that the natural radioactivity in drinking waters from the industrial site was within the prescribed limits and no trend was observed with increasing distance from the IREL site and the distribution was disseminated as evident from the contour plots. The wide variation and some of the higher value may be attributed to the heterogeneous distribution of localized placer deposits of the study area [8, 31, 32].
Effective dose estimation
Effective dose due to drinking water ingestion for adult population (>17 years) can be computed [34] by using the yearly intake of drinking water (4.05 l/d) and IAEA, Basic Safety Series (BSS) dose conversion factors (DCF) for the concerned radionuclides [56].
DCF for 238U, ingestion route = 4.5 × 10−8 Sv/Bq
DCF for 226Ra, ingestion route = 2.8 × 10−6 Sv/Bq
DCF for 210Po, ingestion route = 1.2 × 10−6 Sv/Bq
DCF for 210Pb, for ingestion route = 6.9 × 10−7 Sv/Bq
Mass concentration of natural uranium observed in drinking water can be expressed in activity concentration using a conversion factor of approximately 25 Bq/mg assuming natural uranium composition. Annual committed effective dose can be computed using the formula;
The mean and range of the ingestion dose due to U, 226Ra, 210Po and 210Pb to adult population residing around OSCOM, Odisha from drinking water sources is given in Table 5. The mean value of total effective dose to member of public due to these radioanuclides via drinking water ingestion was found to 67.1 µSv/y with a range of 13.2–198.5 µSv/y. The individual contribution of radioanuclides to the total dose is shown in pie chart (Fig. 5). The main contributor towards the total dose received by the population from drinking water intake is from 226Ra ingestion (65 %). A similar trend was observed in a previous study of the same region [48]. The global annual ingestion dose fall in the range of 200–1,000 µSv/y with a mean of 290 µSv/y [1]. The effective dose received from the present study due to drinking water intake is well within the range of UNSCEAR reported values [1].
Conclusion
A thorough knowledge of the radionuclide concentration in various environmental matrices is imperative in perspective of radiation dose assessment to public. The reason for wide variation of activity concentration in the present study area may be due to heterogeneous distribution of thorium which arises mainly due to monazite placer deposit in the beach sand. The mean value of total effective dose to member of public due to these alpha emitting radionuclides via drinking water ingestion was found to 67.1 µSv/y with a range of 13.2–198.5µSv/y. considering the spatial distribution of radionuclides, no trend was observed with increasing distance from the IREL site and the distribution was disseminated. From the present study it can be concluded that the drinking water which is mostly the ground water (tube well) is not affected by the beach sand mining industry and the activity concentration is due to natural distribution of radioanuclides which is safe for human consumption considering the NORM present therein.
References
UNSCEAR (2008) Sources and effects of ionising radiation. United Nations, New York
Isam Salih MM, Pettersson HBL, Lund E (2002) Uranium and thorium series radionuclides in drinking water from drilled bedrock wells: correlation to geology and bedrock radioactivity and dose estimation. Radiat Prot Dosim 102:249–258
Hakam OK, Choukri AJL, Reyss M Lferde (2001) Determination and comparisons of uranium and radium isotopes activities and activity ratios in samples from some natural water sources in Morocco. J Environ Radioact 57:175–189
Palmer MR, Edmond JM (1993) Uranium in river water. Geochim Cosmochim Acta 57:4947–4955
Ivanovich M, Harmon RS (1992) Uranium series disequilibrium: application to earth marine and environmental sciences. Clarendon Press, Oxford
Cowart JB, Osmond JK (1977) Uranium isotopes in groundwater: their use in prospecting for sandstone-type uranium deposits. J Geochem Explor 8:365–379
Salonen L (1994) 238U series radionuclides as a source of increased radioactivity in groundwater originating from Finnish bedrock, future groundwater resources at risk. IAHS Publ 222:71–84
Mohanty AK, Sengupta D, Das SK, Saha SK, Van KV (2004) Natural radioactivity and radiation exposure in the high background area at Chhatrapur beach placer deposit of Orissa, India. J Environ Radioact 75:15–33
Cullen TL (1977) A review of Brazilian investigations in areas of high natural radioactivity, Part I: Radiometric and dosimetric studies. In: Cullen TL, Penna Franca E (eds) Proceedings of international symposium on areas of high natural adioactivity, Rio de Janeiro, Brazil, 1975. Academia Brasileria De Ciencias, pp 49–64
Penna Franca E (1977) Review of Brazilian investigations in areas of high natural radioactivity, Part II: Internal exposure and cytogenetic survey. In: Cullen TL, Penna Franca E (eds) Proceedings of international symposium on high natural radioactivity, Rio de Janeiro, Brazil, 1975. Academia Brasileria De Ciencias, pp 29–48
Bennett BG (1997) Exposure to natural radiation worldwide. In: Proceedings of the fourth international conference on high levels of natural radiation: radiation doses and health effects, Beijing, China. 1996 Elsevier, Tokyo, pp 15–23
Veiga L, Amaral E, Magalhaes M (1999) Brazilian areas of elevated levels of natural radiation: a critical review and relevant future studies. In: Second symposium on technologically enhanced natural radiation, Rio de Janeiro
Paschoa AS (2000) More than forty years of studies of natural radioactivity in Brazil. Technology 7(193):212
Wei L, Zha Y, Tao Z, He W, Chen D, Yuan Y (1993) Epidemiological investigation in high background radiation areas in Yangjiang, China. In: Proceedings of the international conference on high levels of natural radiation areas, Ramsar, Iran, 1990. IAEA Publication Series IAEA, Vienna, pp 523–547
Wei L, Sugahara T (2000) An introductory overview of the epidemiological study on the population at the high background radiation areas in Yangjiang, China. J Radiat Res 41:1–7
Sunta CM, David M, Abani MC, Basu AS, Nambi KSV (1982) Analysis of dosimetry data of high natural radioactivity areas of southwest coast of India. In: Vohra KG et al (eds) The natural radiation environment. Wiley Eastern Limited India, New Delhi, pp 35–42
Sunta CM (1993) A review of the studies of high background radiation areas of the S–W coast of India. In: Proceedings of the international conference on high levels of natural radiation areas, Ramsar, Iran, 1990. IAEA Publication Series IAEA, Vienna, pp 71–86
Mishra UC (1993) Exposure due to the high natural radiation background and radioactive springs around the world. In: Proceedings of the international conference on high level natural radiation areas, Ramsar, Iran, 1990. IAEA Publication Series IAEA, Vienna, p 29
Paul AC, Pillai PMB, Haridasan P, Radhakrishnan S, Krishnamony S (1998) Population exposure to airborne thorium at the high natural radiation areas in India. J Environ Radioact 40(251):259
Sohrabi M (1993) Recent radiological studies of high level natural radiation areas of Ramsar. In: Proceedings of the international conference on high levels of natural radiation areas, Ramsar, Iran, 1990. IAEA Publication Series IAEA, Vienna
Ghiassi-nejad M, Mortazavi SMJ, Cameron JR, Niroomand-rad A, Karam PA (2002) Very high background radiation areas of Ramsar, Iran: preliminary biological studies. Health Phys 82(87):93
NCRP (1987) Exposure of the population of the United States and Canada from natural background radiation. Report No. 94. National Council on Radiation Protection and Measurements, Bethesda
Radhakrishna AP, Somasekarapa HM, Narayana Y, Siddappa K (1993) A new natural background radiation area on the southwest coast of India. Health Phys 65(390):395
Kannan V, Rajan MP, Iyengar MAR, Ramesh R (2002) Distribution of natural and anthropogenic radionuclides in soil and beach sand samples of Kalpakkam (India) using hyper pure germanium (HPGe) gamma ray spectrometry. Appl Radiat Isot 57(109):119
Sohrabi M (1998) The state-of-the-art on worldwide studies in some environments with elevated naturally occurring radioactive materials (NORM). Appl Radiat Isot 49(169):188
Navratil T, Minarik L (2005) Trace elements and contaminants. www.gli.cas.cz
Gavrilescu ML, Pavel V, Cretescu I (2009) Characterization and remediation of soils contaminated with uranium. J Hazard Mater 163:475–510
Sahoo SK, Mohapatra S, Patra AC, Sagar DV, Tripathi SK, Patra P, Tripathi RM, Puranik VD (2009) Natural radiation and dose rate around thorium mining facility at Orissa, India. International conferences on Recent Advances in Environmental Protection RAEP, Agra, pp 21–22
Mohanty AK, Das SK, Van KV, Sengupta D, Saha SK (2003) Radiogenic heavy minerals in Chhatrapur beach placer deposit of Orissa, southeastern coast of India. J Radioanal Nucl Chem 258:383–389
IBM (Indian Bureau of Mines) (1997) Indian Minerals Year Book 2. Nagpur, Indian Bureau of Mines, p 394
Gusain GS, Rautela BS, Sahoo SK, Ishikawa T, Prasad G, Omori Y, Sorimachi A Tokonami, Ramola RC (2012) Distribution of terrestrial gamma radiation dose rate in the eastern coastal area of Odisha, India. Radiat Prot Dosim 1:4. doi:10.1093/rpd/ncs148
Mohanty AK, Sengupta D, Das SK, Vijayan V, Saha SK (2004) Natural radioactivityin the newlydiscovered high background radiation area on the eastern coast of Orissa, India. Radiat Meas 38(153):165
IAEA (1989) International Atomic Energy Agency Measurement of radionuclides in food and environment. Technical Reports Series no. 295. IAEA, Vienna
Sahoo SK, Mohapatra S, Chakrabarty A, Sumesh CG, Jha VN, Tripathi RM, Puranik VD (2009) Distribution of uranium in drinking water and associated age-dependent radiation dose in India. Radiat Prot Dosim 136:108–113
Rathore DPS, Tarafder PK, Kayal M, Manjeet Kumar (2001) Application of a differential technique in laser-induced fluorimeter: simple and a precise method for the direct determination of uranium in mineralized rocks at the percentage level. Anal Chim Acta 434:201–208
Harada K, Burnett WC, LaRock PA, Cowart JB (1989) Polonium in Florida groundwater and its possible relationship to the sulfur cycle and bacteria. Geochim Cosmochim Acta 53:143–150
Ralph L, Seiler (2011) 210Po in Nevada groundwater and its relation to gross alpha radioactivity. Ground Water 49:160–171
IAEA (2009) Analytical quality in nuclear applications series no. 12. IAEA, Vienna
Mohapatra S, Sahoo SK, Dubey JS, Lenka P, Tripathi RM, Puranik VD (2012) Optimization of operating parameters for low level measurement of alpha emitters by alpha spectrometry. IARP-2012, Trombay, pp 54–55
Tripathi RM, Sahoo SK, Mohapatra S, Lenka P, Dubey JS, Puranik VD (2012) Study of uranium isotopic composition in ground water and deviation from secular equilibrium condition. J Radioanal Nuclear Chem. doi:10.1007/s10967-012-1992-7
Dubey JS, Sahoo SK, Lenka P, Mohapatra S, Jha VN, Rana BK, Tripathi RM and Puranik VD (2013) Determination of ratio of total lead and radioactive lead (210Pb) in ground waters of uranium mining area. In: Proceedings of national symposium on environment-18, pp 53–56
Jia G, Belli M, Blasi M, Marchetti A, Rosamilia S, Sansone (2000) U 210Pb and 210Po determination in environmental samples. Appl Radiat Isot 53:115–120
Sahoo SK, Rana BK, Mohapatra S, Dubey JS, Thakur VK, Lenka P, Jha VN, Tripathi RM and Puranik VD (2013) Ingestion dose due to U and 226Ra through drinking water pathway to members of the public residing around Bagjata uranium mining facility. In: Proceedings of national symposium on environment-18, pp 57–59
Currie LA (1968) Limits for qualitative detection and quantitative determination. Anal Chem 40:586–593
Johns FB, Hahn PB, Thome DJ, Bretthauer EW (1979) Radiochemical procedures for analysis of environmental samples. Environmental Monitoring and Support Laboratory EPA, Las Vegas
Sahoo SK, Mohapatra S, Dubey JS, Thakur VK, Lenka P, Patra AC, Jha VN, Ravi PM and Tripathi RM (2013) Radiological characterization of drinking water in Jaduguda uranium mining area and assessment of effective dose to member of public. In: Fifth symposium on nuclear analytical chemistry NAC-V, pp 531–532
Thakur VK, Sahoo SK, Mohapatra S, Krishna NS, Lenka P, Kumar AV, Ravi PM and Tripathi RM (2013) Estimation of age dependent effective dose due to intake of 226Ra from drinking water in Vizag, India. In: Fifth symposium on nuclear analytical chemistry NAC-V, pp 495–496
Lenka P, Sahoo SK, Mohapatra S, Patra AC, Dubey JS, Vidyasagar D, Tripathi RM, Puranik VD (2012) Ingestion Dose from 238U, 232Th, 226Ra, 40K and 137Cs in cereals, pulses and drinking water to adult population in a high background radiation area, Odisha, India. Radiat Prot Dosim. doi:10.1093/rpd/ncs115
Patra AC, Mohapatra S, Sahoo SK, Lenka P, Dubey JS, Thakur VK, Kumar AV, Ravi PM, Tripathi RM (2014) Assessment of ingestion dose due to radioactivity in selected food matrices and water near Vizag, India. J Radioanal Nucl Chem. doi:10.1007/s10967-014-3097-y
Mohapatra S, Sahoo SK, Kumar AV, Patra AC, Lenka P, Dubey JS, Thakur VK, Tripathi RM, Puranik VD (2013) Distribution of NORM and 137Cs in soils of Visakhapatnam region, eastern India and associated radiation dose. Radiat Prot Dosim 157(1):95–104
Sethy NK, Tripathi RM, Jha VN, Sahoo SK, Shukla AK, Puranik VD (2011) Assessment of natural uranium in the ground water around jaduguda uranium mining complex, India. J Environ Prot 2:1002–1007
Othman I, Yassine T (1996) Natural radioactivity of drinking water in the southern and middle parts of Syria. Environ Int 22:S355–S359
WHO (2011) World Health Organization guidelines for drinking water quality, 4th edn. WHO, Geneva
USEPA (2000) United States Environmental Protection Agency national primary drinking water regulations. Radionuclides final rule 40 CFR parts 9: 141, and 142
AERB (2004) Atomic Energy Regulatory Board Limit for uranium in drinking water, India. AERB, Trombay
BSS (1996) International Atomic Energy Agency. Safety series no. 115. IAEA, Vienna
Tripathi RM, Sahoo SK, Jha VN, Khan AH, Puranik VD (2008) Assessment of environmental radioactivity at uranium mining, processing and tailings management facility at Jaduguda, India. Appl Radiat Isot 66:1666–1670
Rani A, Singh S (2006) Analysis of uranium in drinking water samples using laser induced fluorimetry. Health Phys 9:101–107
Bomben AM, Equillor HE, Oliveira AA (1996) Ra-226 and natural uranium in Argentina bottled mineral waters. Radiat Prot Dosim 67:221–224
Alam MN, Chowdhury MI, Kamal M, Ghose S, Islam MN, Anwaruddin M (1999) Radiological assessment of drinking water of the Chittagong region of Bangladesh. Radiat Protect Dosim 82:207–214
Jde Oliveira, Paci Mazzilli B, Da Costa P, Akiko Tanigava P (2001) Natural radioactivity in Brazilian bottled mineral waters and consequent doses. J Radioanal Nuclear Chem 249:173–176
Jde Oliveiraa, Paci Mazzilli B, Oliveira Sampa MHde, Bambalas E (2001) Natural radionuclides in drinking water supplies of SaÄ o Paulo State Brazil and consequent population doses. J Environ Radioact 53:99–109
Vesterbacka P, Turtiainen T, Heina¨vaara S, Arvela H (2006) Activity concentrations of 226Ra and 228Ra in drilled well water in finland. Radiat Prot Dosim 121:406–412
Vesterbacka P, Ma¨kela¨inen I, Arvela H (2005) Natural radioactivity in drinking water in private wells in Finland. Radiat Protec Dosim 113:223–232
Jobbágy V, Kávási N, Somlai J, Máté B, Kovács T (2010) Radiochemical characterization of spring waters in Balaton Upland Hungary estimation of radiation dose to members of public. Microchem J 94:159–165
Desideri D, Meli MA, Feduzi L, Roselli C, Rongoni A, Saetta D (2007) 238U, 234U, 226Ra, 210Po concentrations of bottled mineral waters in Italy and their dose contribution. J Environ Radioact 94:86–97
Villalba L, Montero-Cabrera ME, Manjo´n-Collado G, Colmenero-Sujo L, Renterı´a-Villalobos M, Cano-Jime´nez A, Rodrı´guez-Pineda A, Da´vila-Rangel I, Quirino-Torres L, Herrera-Peraza EF (2006) Natural radioactivity in groundwater and estimates of committed effective dose due to water ingestion in the state of Chihuahua (Mexico). Radiat Prot Dosim 121:148–157
Ontario Ministry of Environment and Energy (1996) Monitoring data for uranium—1990–1995. Toronto, Ontario, OMEE Ontario Drinking Water Surveillance Programme, Ontario
Walencik A, Kozowska B, Dorda J, Zipper W (2010) Natural radioactivity in underground water from the outer Carpathians in Poland with the use of nuclear spectrometry techniques. Appl Radiat Isot 68:839–843
Osman AAA, Salih I, Ibrahim A, Shaddad El Din S, Siddeeg MB, Eltayeb H, Idriss H, Hamza W, Yousif EH (2008) Investigation of natural radioactivity levels in water around Kadugli, Sudan. Appl Radiat Isot 66:1650–1653
Bakac M, Kumru MN (2000) Uranium, radium and field measurements in the water of Gediz river. Turk J Eng Environ Sci 24:229–236
Kumru MN (1995) Distribution of radionuclides in sediments and soils along the Bu¨yu¨k Menderes river. Proc Pak Acad Sci 32:51–56
Acknowledgments
Authors gratefully acknowledge the guidance and encouragement of Dr. D. N. Sharma, Director, Health Safety and Environment Group, BARC. The help and support received from the colleagues of Health Physics Units, Orissa Sands Complex, and the authorities of IREL during environmental sampling and survey of the study area is greatly acknowledged by the authors. The whole heartedly contribution of Dr. Manoj Mohapatra, Radiochemistry Division, BARC is greatly acknowledged during sampling and survey of the study area. Authors are truly grateful for the cooperation received from other colleagues of the lab during the progress of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohapatra, S., Sahoo, S.K., Dubey, J.S. et al. Characterization of uranium and its progenies in drinking water and assessment of dose to public around a NHBRA, Odisha, India. J Radioanal Nucl Chem 303, 601–613 (2015). https://doi.org/10.1007/s10967-014-3353-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3353-1