Abstract
A new supermolecular recognition agent named tetra-tert-butyl-calix[4]-bis[(4-methly-1,2-phenylene-crown-6] (TertButylCalixC) was synthesized. Batchwise experiments of extraction of Na(I), K(I), Rb(I), Cs(I), Sr(II), Ba(II), Ru(III) and Fe(III) from aqueous solution with TertButylCalixC/o-nitroanisole were carried out. Effects of HNO3 concentration, contact time and temperature on the extraction behavior were examined. The equilibrium established within 30 min implied a fast extraction kinetics. The optimal operation temperature was 298 K. Stoichiometry of TertButylCalixC and Cs(I) by electrospray ionization mass spectrometry characterization revealed 1:1 molar ratio. It was observed that the extraction system of TertButylCalixC/o-nitroanisole was effective for selective removal of Cs(I), showing remarkable separation performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calix[4]crowns refer to the compounds in which calix[4]arenes are combined with crowns through the bridging of phenolic oxygens of calix[4]arenes. Due to their superior recognition ability toward alkali metal cations and other ions [1–6], calix[4]crowns have received increasing attention. Calix[4]crowns have been found to show the potential application in the fields of molecular sensors [7], ion selective electrodes [8], artificial allosteric systems [9], separation and purification [10–12]. According to the number of polyether bridging on the lower rim of a calix[4]arene molecule, calix[4]crowns can be divided into two types. One is calix[4]monocrown. The other is calix[4]biscrown. Both two types of calix[4]crowns, especially calix[4]crown-6 in 1,3-alternate conformation, are taken seriously due to their high selectivity for Cs(I)/Na(I) via matching size and π-interaction [13–15].
Due to the high selectivity for Cs(I)/Na(I), 1,3-alternate calix[4]biscrown-6 has been paid more attention than 1,3-alternate calix[4]monocrown-6 in recent years [16, 17]. Processes like fission product extraction [18, 19] and caustic-side solvent extraction [20, 21] using calix[4]biscrowns to selectively separate cesium from the highly active liquid waste (HLW) have been reported. The Cs(I)/Na(I) selectivity of calix[4]-bis-crown-6 could reach a level of 1,500. The selectivities of calix[4]-bis-benzo-crown-6 and calix[4]-bis-naphtho-crown-6 were more than 19,000 and 29,000, respectively. Although the calix[4]biscrown-6 derivative had remarkable Cs(I)/Na(I) selectivity, the Cs(I) extraction ability was not high. The distribution ratios of Cs(I) reported were not greater than 10 [22], which would result in the increment of extraction stage for better separation. However, in contrast to the extensive investigations on the host molecule of calix[4]arene, very little is known about tetra-tert-butyl-calix[4]arene.
Two major radioisotopes of cesium, 135Cs (t 1/2 = 1.0 × 105 years) and 137Cs (t 1/2 = 30 years), can be produced from nuclear reactors. The two radioisotopes remain in HLW, the raffinate of PUREX which works for the recovery of uranium and plutonium from the spent nuclear fuel. Removal of 135Cs and 137Cs is necessary as their radioactivity brings adverse effect on environment and the treatment of HLW [23–25]. 137Cs is also a heat generator element and hampers the final vitrification of HLW [26]. As a β-emitter, 137Cs can be applied in several industries [27, 28]. Efforts are on to separate cesium from HLW, but the effective separation is problematic. One problem is lack of an applicable extractant.
Tetra-tert-butyl-calix[4]arene containing Tetra-tert-butyl groups is a derivative of calix[4]arene. It might has more strong supramolecular recognition ability than calix[4]arene. Furthermore, tetra-tert-butyl groups make tetra-tert-butyl-calix[4]arene more lipophilic than calix[4]arene. This is of great beneficial to its dissolution in alkane diluents.
In the present work, a new calix[4]biscrown derivative, tetra-tert-butyl-calix[4]-bis[(4-methly-1,2-phenylene-crown-6] (TertButylCalixC), was synthezied by a cyclization reaction. Following TertButylCalixC was identified by proton nuclear magnetic resonance (1H NMR), electrospray ionization mass spectrometry (ESI–MS), elemental analysis, and others. The solvent extraction of Cs(I) and other typical metals with TertButylCalixC/o-nitroanisole in the concentration range of 0.4 M to 6.0 M HNO3 was carried out. The main factors affecting the extraction properties such as the HNO3 concentration, contact time, and temperature were investigated. The extraction mechanism and properties of the tested metals were discussed.
Experimental
Materials
All the chemicals and reagents used in the study were of analytical grade, unless otherwise specified. Ruthenium nitrosyl nitrate solution (1.5 wt% of Ru(III)) was procured from the Strem Chemicals, the United States of America. The other metal nitrates were purchased from the Minxing Co. Inc. China. Because the electron-deficiency solvents such as CHCl3, CH2Cl2 and others usually can be effectively associated with calix[4]biscrown through hydrogen bonding, the available concentration of calix[4]biscrown in organic phase decreased. It makes the extraction ability of Cs(I) with calix[4]biscrown decreased significantly. An electron-donor organic solvent, o-nitroanisole provided by the Aladdin company, was therefore utilized as a diluent. Tetra-tert-butyl-calix[4]arene and bis[1,2-[2′(2″-hydroxy-ethoxy)ethoxy]]-4-methylbenzene di-p-toluenesulfonate used for the synthesis of TertButylCalixC was prepared.
Considering the toxicity of o-nitroanisole, all experiments were performed in an airtight glovebox, while an antigas mask was utilized to health protection.
A calix[4]biscrown supermolecular derivative, tetra-tert-butyl-calix[4]-bis[(4-methly-1,2-phenylene-crown-6] (TertButylCalixC), was prepared quantitatively synthesized through an improved experimental method.
Apparatus
1H NMR spectrum was recorded in an Avance DMX500 (Bruker, Switzerland) spectrometer. Mass spectra was obtained with a PerkinElmer SciexAPI 3000 (PerkinElmer, America) electrospray ionization mass spectrometry. Elemental analysis was carried out with an X'Pert PRO X (PANalytical, Netherland). The metal ion concentration was determined by using a Varian AA 240 FS model atomic adsorption spectroscope.
Preparation of TertButylCalixC
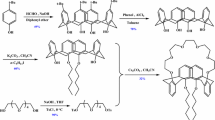
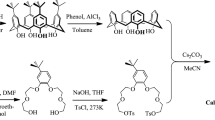
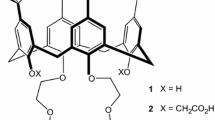
TertButylCalixC was synthesized according to a classical method [29]. Under a condition of N2 tetra-tert-butyl-calix[4]arene (1) and Cs2CO3 were mixed in acetonitrile and stirred at reflux for 2 h. Then bis[1,2-[2′(2″-hydroxyethoxy)ethoxy]]-4-methylbenzene di-p-toluene sulfonate (2) was added dropwise and the mixture was stirred at reflux for 20 days. After cooling, the solvent was removed in vacuo. Chloroform was added and the mixture was neutralized by dilute hydrochloric acid solution. The organic layer was separated and dried over magnesium sulfate. The filtered solution was concentrated under vacuo and the residue was purified by column chromatography [SiO2, petroleum ether-ethyl acetate (25:1)] to yield TertButylCalixC. 1H NMR (300 MHz, CDCl3, δ relative to TMS) ppm: 1.23 (s, 36H, C(CH 3)3), 2.30 (s, 6H, CH 3), 2.86–3.68 (m, 24H, OCH 2CH 2O), 3.97 (s, 8H, ArCH 2Ar), 3.96–4.02 (m, 8H,OCH 2CH 2O), 6.66–6.79 (m, 6H, ArHbenzo), 7.02 (s, 8H, ArHcalix). MS (ESI) m/z: 1,177.9 [M + H]+. Elemental analysis found: 75.25 % for C, 8.46 % for H. calcd: 75.48 % for C, 8.22 % for H. Figure 1 shows the scheme of synthesis of TertButylCalixC.
Solvent extraction studies
All extraction experiments were conducted batch wise in duplicate. Prior to an extraction experiment, the organic phase with 8.0 × 10−3 M of TertButylCalixC in o-nitroanisole was preconditioned via one contact with equal volume of nitric acid solution without metal ions. Equal volume (5.0 cm3) of the aqueous phase containing 5.0 × 10−4 M of Na(I), K(I), Rb(I), Cs(I), Sr(II), Ba(II), Ru(III) and Fe(III) ions and the organic phase were shaken at 120 rpm for the required time in a conical flask. The phases were allowed to settle down and centrifuged for disengagement. Errors in the measurement of the concentration of the tested metals by the atomic adsorption spectroscope in aqueous phase was within ±0.5 %. The distribution ratio (D) was calculated by taking the ratio of equilibrium concentration of metal ion in the organic phase and that in the aqueous phase after extraction. The equation for D was listed by Eq. (1).
where C (o) and C (a) denote the equilibrium concentration of the tested metals in organic phase and aqueous phase after extraction, respectively.
Separation factor of Cs(I) to the other tested metals, SF Cs/M, was figured out by Eq. (2).
Results and discussion
Effect of contact time on the extraction
Datum about distribution ratios at different contact time is useful for the determination of equilibrium as well as for a better separation at non equilibrium if needed. Therefore the investigation on the effect of contact time on extraction is meaningful. These typical fission and nonfission elements, Na(I), K(I), Rb(I), Cs(I), Sr(II), Ba(II), Ru(III) and Fe(III) were extracted by TertButylCalixC/o-nitroanisole for different contact time at 4.0 M HNO3 medium. The results are shown in Fig. 2.
It was found that the increase in contact time in 30 min increased D Cs quickly. Values of D Cs were 0.82 at 1 min, 7.26 at 5 min, 8.06 at 10 min, 15.39 at 20 min and 16.09 at 30 min, respectively. A further increase in contact time up to 240 min had a negligible effect on D Cs which kept at a constant value ca. 15.61. The nature of fast extraction dynamic was proved. The explanation for this was that the process of recognization of TertButylCalixC toward Cs(I) was fast and the resultant complex was transferred into the organic phase quickly. For the other tested elements, only Rb(I) had a similar extraction dynamic process with Cs(I) and the distribution ratio of Rb(I) at equilibrium was nearly 1.86. Na(I), K(I), Sr(II), Ba(II), Ru(III) and Fe(III) ions showed almost no extraction due to the weak complexation.
Comparing with extraction systems based on other calixbiscrowns, the TertButylCalixC/o-nitroanisole system had a good cesium extraction ability. The maximum distribution ratio of Cs(I) in the TertButylCalixC/o-nitroanisole extraction system reached round 16 which is significantly greater than distribution ratios in literatures [22]. The distribution ratios of TertButylCalixC and some typical calix[4]biscrown derivatives in the extraction of Cs(I) are listed in Table 1.
It could be concluded that under the experimental conditions the TertButylCalixC/o-nitroanisole system had high ability to selectively extract Cs(I). The equilibrium of extraction of TertButylCalixC/o-nitroanisole toward Cs(I) reached in 30 min.
Effect of HNO3 concentration on the extraction
Highly active liquid waste, the raffinate of PUREX process, contains a high amount of nitric acid. Hence, it is necessary to investigate the effect of HNO3 concentration on the solvent extraction with TertButylCalixC/o-nitroanisole. The evaluation experiment was carried out and 240 min of shake time was utilized to make the extraction equalized. The results are shown in Fig. 3.
As can be seen, the distribution ratio of Cs(I) (D Cs) increased from 2.68 to 16.82 with increasing HNO3 concentration from 0.4 to 4.0 M. The reason for this was that the reaction A (Fig. 4) was dominant in extraction system. D Cs decreased from 16.82 to 13.55 with increasing HNO3 concentration from 4.0 to 6.0 M. The reason for this behavior was that the coordination reaction B (Fig. 4) between HNO3 and TertButylCalixC was the main reaction which decreased the effective quantity of TertButylCalixC for Cs(I) extraction. The coordination reaction resulted from oxygen atoms in crown ether moieties, which had strong affinity toward nitric acid through hydrogen bonding.
Furthermore, D Cs were obviously greater than D of the other metals in the range of 0.4 M to 6.0 M HNO3 concentration. For all the other metals, only Rb(I) was slightly extracted with a maximum distribution ratio of 1.94 because the properties of Rb(I) was the most close to them of Cs(I). Therefore good selectivity for Cs(I) over the other metals was affirmed. The process of good selectivity for Cs(I) could be described as follows. When a TertButylCalixC molecule was surrounded by Na(I), K(I), Rb(I), Cs(I), Sr(II), Ba(II), Ru(III) and Fe(III) ions, the TertButylCalixC molecule only accepted Cs(I) by an oxygen-rich cycle due to the similar size. The accepted Cs(I) ion was fixed in the oxygen-rich cycle by lone pair electrons of oxygen atoms. Then the resultant complex was transferred into the organic phase by dissolution.
Separation factors of Cs(I) to the other metals at the evaluated HNO3 concentration were worked out and shown in Table 2.
According to above results, it was concluded that the extraction system based on the new compound TertButylCalixC had selective extraction ability toward Cs(I). Other metals such as Na(I), K(I), Rb(I), Sr(II), Ba(II), Ru(III) and Fe(III) ions almost had no influence on the selectivity. The optimum HNO3 concentration for the extraction was 4.0 M which was close to the acidity of actual HLW.
Effect of temperature on the extraction
To understand the thermodynamic behavior, the extraction of Na(I), K(I), Rb(I), Cs(I), Sr(II), Ba(II), Ru(III), and Fe(III) with the TertButylCalixC/o-nitroanisole system in 4.0 M HNO3 was conducted at temperatures of 298, 303, 308, 313, and 318 K. The relevant results are shown in Fig. 5.
As shown in Fig. 5, D Cs sharply decreased at higher temperatures, which indicated that extraction of Cs(I) in this system was an exothermic process. Other metals showed negligible extraction in the evaluated temperature range except for Rb(I), which presented similar tendency with Cs(I). It is known that both Rb(I) and Cs(I) are alkali metals and their ionic radius are close. This makes it possible that as TertButylCalixC recognizes Cs(I) ion, the size of Rb(I) might also match with cavity of TertButylCalixC. It resulted in the effective recognition of TertButylCalixC for Rb(I) when Cs(I) was extracted. The solvent extraction behavior of Rb(I) with TertButylCalixC was therefore similar to that of Cs(I). Thermodynamics parameters of the extraction reaction such as Gibbs free energy change (ΔG°), enthalpy change (ΔH°), and entropy change (ΔS°) were calculated by Arrhenius law dlnD/dT = ΔH°/(RT 2) and Gibbs free energy ΔG° = ΔH° − TΔS°. The corresponding results are listed in Table 3. The negative ΔG° of Cs(I) extraction at all evaluated temperature confirmed the feasibility of the process and spontaneous nature. The decrease of the value of ΔG° with increasing temperature indicated that the recognition of TertButylCalixC toward Cs(I) was weaker. The negative value of ΔH° (−43.2 kJ·mol−1) of Cs(I) extraction confirmed the extraction reaction was exothermic. The negative value of ΔS° (−121.28 J mol−1 K−1) of Cs(I) extraction reflected a decrease in randomness. For Rb(I), the negative value of ΔH° (−94.84 kJ mol−1) and ΔS° (−309.94 J mol−1 K−1) also denoted the extraction was exothermic and a decrease in randomness. All of these results showed that in HNO3 solution, Cs(I) and Rb(I) had similar extraction properties due to close ionic radius and electric structure.
The discussion of the extraction mechanism
Electrospray ionization mass spectrometry is regarded as a powerful method for chemical stoichiometry determination. To investigate the extraction mechanism, an extraction experiment identical to the extraction above was performed except the aqueous phase only contained Cs(I). The separated organic phase was then characterized by ESI–MS. The result is shown in Fig. 6.
The peak of 1,194.0 was determined as the complex of [TertButylCalixC·NH4]+. The strongest mass peak at 1,309.0 indicated the formation of 1:1 mononuclear complex, [TertButylCalixC·Cs]+. The mass peak at 1,443.2 was considered to be [TertButylCalixC·2Cs]2+, proving the existence of the 1:2 binuclear complex. However, the extent of formation of the 1:2 binuclear complex was a hundred-fold less than formation of the 1:1 mononuclear complex. The results demonstrated that 1:1 mononuclear complex accounted for the mechanism of extraction. Since calix[4]biscrown-6 had the same complex behavior [29], it could be concluded that the analogue of calix[4]biscrown tended to form 1:1 mononuclear complex with Cs(I) rather than 1:2 binuclear complex. The reason for this was that the occupation of one crown cycle in the TertButylCalixC molecule by one Cs(I) ion resulted in the loss of complex ability of the other crown cycle toward Cs(I). The possible complex is shown in Fig. 7.
Conclusions
Calixbiscrowns appear to be suitable extractants for the removal of cesium from HLW due to their recognization ability toward cesium. The optimum calixbiscrown for cesium separation is still unknown. With this in mind, a new calixbiscrown derivative named TertButylCalixC was successfully fabricated. The extraction of Na(I), K(I), Rb(I), Cs(I), Sr(II), Ba(II), Ru(III) and Fe(III) ions from aqueous solution with TertButylCalixC/o-nitroanisole was conducted. TertButylCalixC/o-nitroanisole could selectively extract Cs(I). The extraction of Cs(I) with TertButylCalixC/o-nitroanisole depended on the HNO3 concentration. The extraction process reached equilibrium within 30 min. Increasing temperature was adverse to the extraction and the suitable temperature was 298 K. ESI–MS preliminarily determined a 1:1 mononuclear complex was formed. The extraction properties and mechanism of the tested metals were discussed. The thermodynamic data for the solvent extraction Cs(I) and Rb(I) were obtained. TertButylCalixC/o-nitroanisole was proved to be effective for the separation of Cs(I).
References
Mokhtari B, Pourabdollah K (2012) Desalination 292:1–8
Mokhtari B, Pourabdollah K (2012) J Chin Chem Soc-Taip 59:1058–1069
Mokhtari B, Pourabdollah K (2012) J Electrochem Soc 159:K61–K65
Mokhtari B, Pourabdollah K (2012) Electrochim Acta 76:363–367
Mokhtari B, Pourabdollah K (2012) J Incl Phenom Macro Chem 73:269–277
Mokhtari B, Pourabdollah K (2012) Electroanal 24:219–223
Bouhroum S, Arnaud-Neu F, Asfari Z, Vicens J (2004) Russ Chem Bull 53:1544–1548
Mokhtari B, Pourabdollah K, Dalali N (2011) Chromatographia 73:829–847
Saadioui M, Asfari Z, Vicens J (1997) Tetrahedron Lett 38:1187–1190
Mokhtari B, Pourabdollah K, Dalali N (2011) J Radioanal Nucl Chem 287:921–934
Mokhtari B, Pourabdollah K, Dalali N (2011) J Incl Phenom Macro Chem 69:1–55
Mokhtari B, Pourabdollah K (2012) J Incl Phenom Macro Chem 73:1–15
Haverlock TJ, Bonnesen PV, Sachleben RA, Moyer BA (2000) J Incl Phenom Macro Chem 36:21–37
Raut DR, Kandwal P, Rebello G, Mohapatra PK (2012) J Membr Sci 407:17–26
Raut D, Mohapatra P, Ansari S, Manchanda VK (2009) Sep Sci Technol 44:3664–3678
Zhou H, Connery KE, Bartsch RA, Moyer BA, Haverlock TJ, Delmau LH (2013) Solvent Extr Ion Exch 31:683–696
Nakase M, Kinuhata H, Takeshita K (2013) J Nucl Sci Technol 50:1089–1098
Riddle CL, Baker JD, Law JD, McGrath CA, Meikrantz DH, Mincher BJ, Peterman DR, Todd TA (2005) Solvent Extr Ion Exch 23:449–461
Mincher B, Mezyk S, Bauer W, Elias G, Riddle C, Peterman D (2007) Solvent Extr Ion Exch 25:593–601
Pierce R, Peters T, Crowder M, Caldwell T, Pak D, Fink S, Blessing R, Washington A (2011) Demonstration of the next-generation caustic-side solvent extraction solvent with 2-cm centrifugal contractors using tank 49H waste and waste simulant, SRNL-STI-2011-00589. Savannah River National Laboratory, Aiken
Delmau LH, Haverlock TJ, Bazelaire E, Bonnesen PV, Ditto ME, Moyer BA (2009) Solvent Extr Ion Exch 27:172–198
Sachleben RA, Bonnesen PV, Descazeaud T, Haverlock TJ, Urvoas A, Moyer BA (1999) Solvent Extr Ion Exch 17:1445–1459
Lahtz C, Bates SE, Jiang Y, Li AX, Wu X, Hahn MA, Pfeifer GP (2012) PLoS One 7:e44858
Cho IJ, Park SY, Ko WI, Kim YS, Park BH (2013) J Comput Theor Nanosci 10:1714–1721
Fokin A, Torshin S (2013) Eurasian Soil Sci 46:386–392
Mohapatra P, Lakshmi D, Bhattacharyya A, Manchanda VK (2009) J Hazard Mater 169:472–479
Kumar V, Sharma JN, Achuthan PV, Hubli RC (2014) J Radioanal Nucl Chem 299:1547–1553
Sun B, Hao XG, Wang ZD, Guan GQ, Zhang ZL, Li YB, Liu SB (2012) J Hazard Mater 233–234:177–183
Gao JX, Wang JC, Song CL, Liu T, Hu TD, Xie YN, Zhang J, Wang G, Yang H (2006) J Solut Chem 35:113–119
Acknowledgments
The present work was financially supported by the National Natural Science Foundation of China under contract No. 91126021, Zhejiang Provincial Natural Science Foundation of China under contract No. Y4110002, and Zhejiang Provincial Commonweal Technology Applied Research Project under contract No. 2012C23032.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, Y., Zhang, A. Extraction equilibrium and thermodynamics of cesium with a new derivative of calix[4]biscrown. J Radioanal Nucl Chem 302, 575–581 (2014). https://doi.org/10.1007/s10967-014-3287-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3287-7