Abstract
To elucidate the sorption behaviors of radionuclides in multi-mineral systems and the mutual effects of minerals on this sorption, we carried out the sorption and desorption experiments of neptunium(V) on Na-montmorillonite-based two-mineral phasic systems (montmorillonite–calcite, montmorillonite–apatite). In the montmorillonite–calcite system, the sorption on the montmorillonite moiety decreased with increasing calcite content due to interference by the calcium ions dissolved from the calcite moiety, while no accumulation of Np to the calcite was observed. Total sorption of Np on the montmorillonite–apatite system was larger than that on apatite-free Na-montmorillonite, but the sorption on the montmorillonite moiety in this system was less than that on apatite-free Na-montmorillonite. Under weakly acid and neutral pH conditions, Np accumulated on the apatite moiety in a short period. At final pH 4 or less, though the pH condition was sufficient to dissolve the apatite moiety completely, the sorption very slowly increased with time and the increased Np was unexchangeable with 1 M KCl solution. This increase of the unexchangeable sorption cannot be explained by the knowledge accumulated so far.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Extensive research has been made on migration behaviors of radionuclides released by potential and actual events (degradation of radioactive wastes and accident of nuclear facilities) to the subsurface environment. Sorption is one of the key mechanisms for controlling the subsurface environmental behavior of radionuclides. The sorption of radionuclides on solid phases includes ion exchange, complexation, physical adhesion, and subsequent precipitation and mineralization. In most of the relevant experimental studies, sorption experiments of radionuclides have been performed in a single-mineral system, while most of the subsurface environment is a multiphasic system. Knowledge of the mutual influence of minerals on sorption in multiphasic systems is very limited.
To explore the knowledge, this paper investigated the sorption of neptunium(V) in two-mineral systems. Np(V) was chosen because it is one of the radionuclides having low sorptivity for many minerals [1–4]. The oxycation of Np(V), NpO2 +, is stable in a wide pH range under aerobic conditions [5]. The distribution coefficient (K d) of NpO2 + for bentonite clay, which is a candidate material for engineered barrier due to its high cation exchange capacity, is only 10–20 cm3 g−1 at intermediate pH (Kozai et al. [6]) (cf. K ds for Cs+ [7] and Am3+ [8, 9] ≥103 cm3 g−1). Because of its low sorptivity and long half-life (2.1 × 106 year of 237Np), Np, particularly 237Np, is of special importance for safety assessments of high-level radioactive waste disposal.
This paper tested montmorillonite as a major component mineral of two-mineral systems because of its high reactivity with ions and relatively high stability in natural waters. Montmorillonite is a swelling clay mineral found ubiquitously in the soil environment. Due to its moderate negative surface charge and large specific surface area, this clay has high cation exchange ability for many cations. However, the exchange capacity for NpO2 + is low [10]. As minor component minerals, calcite (CaCO3) and apatite (Ca5(PO4)3OH) were selected because of their high reactivities with ions and low stabilities under acidic conditions. Calcite is one of the most common minerals and comprises about 4 % of the Earth’s crust by weight [11]. Phosphate minerals are not abundant because phosphorous comprises only 0.1 % of the Earth’s crust by weight [12, 13]. The carbonate and phosphate groups respectively contained in those minerals have high abilities to form stable complexes with heavy metals including Np(V). Those two minerals therefore have high abilities to sorb Np(V) [14–16]. Compared to montmorillonite, calcite and apatite are unstable in regard to the environmental changes. Calcite and apatite release their component cations and anions depending on chemical conditions such as pH.
This paper applies a simplified desorption method to the sorption study and discusses the distribution of Np between minerals.
Experimental
A 237Np(V) nitrate stock solution was diluted with a 0.01 M NaClO4 aqueous solution to yield a working solution having a Np concentration of about 6 × 10−7 M.
Tsukinuno montmorillonite (Kunipia F®, Kunimine Industries Co. Ltd., Japan) was used after the montmorillonite was converted to homoionic Na+ form (Na-montmorillonite) using a 1 M NaCl aqueous solution [10]. Natural calcite (CaCO3) was used after being ground to particle sizes below 74 μm. The apatite used was a fine powder of commercial calcium-phosphate having a hydroxyapatite (Ca5(PO4)3OH) like structure (Wako Pure Chemical Industries Co. Ltd., Japan). These minerals were used singly or as montmorillonite-based systems (the content of the montmorillonite was 95–99 wt%).
For the sorption experiments, the initial pH of the Np solution was adjusted with dilute HCl and NaOH aqueous solutions. A weighed amount (0.06 g) of sold phase was soaked in 6 cm3 aliquot of the Np solution and stored at 20 °C. This solid-solution mixture was agitated once a day. After a prescribed period, the liquid phase was collected by centrifugation (10,000 rpm, 1 h) and transferred to another tube. During the sorption experiments, the pH values of the liquid phases changed to final values by the buffering actions of the minerals. This study set the final pH to the range from 4 to 8 where almost all of the Np(V) species are present as NpO2 + [5].
Desorption experiments were conducted in two steps. First, 6 cm3 of a 1 M KCl aqueous solution was added to the solid phase collected after the sorption experiment. After 2 days, the liquid phase was separated from the solid phase by centrifugation and transferred to another tube. Second, 6 cm3 of a 1 M HCl aqueous solution was added to the solid phase. After 2 days, the liquid phase was separated from the solid phase by centrifugation and transferred to another tube. The treatment with each solution was carried out twice to confirm complete desorption of Np. We refer to the Np desorbed with 1 M KCl aqueous solution as “exchangeable” Np and the Np not desorbed with 1 M KCl but with 1 M HCl aqueous solution as “unexchangeable” Np. The total sorption is the sum of the exchangeable sorption and unexchangeable sorption.
The Np concentrations in the liquid phases were measured by the combination of a liquid scintillation analyzer with alpha/beta discrimination (Tri-Carb® 2550TR/AB, Packard Bioscience, USA) and liquid scintillation cocktails (Ultima-Gold AB and F, Packard Bioscience). Concentrations of Ca in the liquid phases were determined with an inductively coupled plasma atomic emission spectrometer (Model SPS1200A, Seiko EG&G, Japan). Concentrations of PO4 3− in the liquid phases were determined with an ion chromatograph analyzer (Model IC7000, Yokogawa Electric Corp., Japan). Powder X-ray diffraction (XRD) data were obtained with an X-ray diffractometer (Ultima IV, Rigaku Corp., Japan). Energy dispersive X-ray (EDX) analysis for solid phases was performed using a scanning electron microscope (SEM) with an X-ray analysis system (Phenom ProX, Phenom-World, The Netherlands).
Results and discussion
Sorption in single mineral systems
Montmorillonite
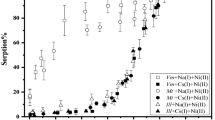
The open circles in Fig. 1 show the total sorption of Np on Na-montmorillonite as a function of final pH after the 10 days sorption experiments. The percentages of the total sorption attained stable values in 10 days [17] and were roughly constant between 20 and 40 % in the tested final pH range except near 8. At this pH, the total sorption was somewhat higher than that at lower pH. As shown by the black circles in Fig. 1 most of the sorbed Np was desorbed from the montmorillonite by twice treatment with 1 M KCl aqueous solutions.
Sorption of Np(V) on Na-montmorillonite, calcite, and apatite as a function of final pH of liquid phases. Part of the data for Na-montmorillonite was quoted from the previous publications (Kozai et al. [6]) and the other data were obtained in the present study
Montmorillonite has a permanent negative charge inside its layered crystal structure [18]. The permanent negative charge is pH-independent and constant. Except at pH near 8 or higher where a pH-dependent charge is generated at the broken edges of the montmorillonite layers, the total negative charge of montmorillonite was constant under the pH conditions of this study. Because the permanent negative charge resides inside the layered structure, the bond between the permanent negative charge and the cations is indirect and thus loose. The cations bound to the montmorillonite are easily substituted for other cations. This knowledge is consistent with the present results. That is, NpO2 + was retained on the montmorillonite by electrostatic attraction with the constant negative charge of the montmorillonite in most of the tested final pH range and the sorbed NpO2 + was desorbed by the exchange with K+. The slight increase of the Np sorption at final pH near 8 is due to the generation of the pH-independent negative charge.
Calcite
Because calcite is easily dissolved in acid solution, the initial pH was restricted to above approximately 4. Under this initial pH range, the final pH exceeded 8 (Fig. 2) due to the partial dissolution of calcite shown below.
Almost all of the Np was sorbed in the examined final pH range (open diamonds in Fig. 1). The Np sorbed on the calcite was hardly desorbed with 1 M KCl aqueous solutions (black diamonds in Fig. 1). Np(V) forms a stable inner-sphere complex with the surface carbonate groups of calcite [16]. This knowledge is consistent with the present results of the high sorptivity of calcite and the high stability of the sorbed Np in 1 M KCl aqueous solution.
Apatite
As shown in Fig. 2, the apatite showed a pH buffering ability similar to that of Na-montmorillonite. At an initial pH below 3, the final pHs were higher than those of Na-montmorillonite and part of the apatite was dissolved (Fig. 3).
Almost all of the Np was sorbed on the apatite (open squares in Fig. 1) in the examined final pH range. The Np sorbed on the apatite was hardly desorbed by twice treatment with 1 M KCl aqueous solutions (black squares in Fig. 1). The sorption of Np(V) on apatite is supposed to be inner-sphere surface complexation with surface phosphate groups, though studies on the sorption of Np(V) on apatite is very limited and the mechanism remains to be investigated [14, 19]. This mechanism is consistent with the present results of the high sorptivity of apatite and the high stability of the sorbed Np in 1 M KCl aqueous solution.
As demonstrated above, there is a clear difference in the desorption behavior of the sorbed Np between montmorillonite and the other two minerals. The subsequent experiments for the montmorillonite-based systems utilized these clear differences; that is, the Np desorbed with 1 M KCl aqueous solutions (exchangeable Np) was regarded as Np sorbed on the montmorillonite moiety, and the Np not desorbed with 1 M KCl aqueous solutions (unexchangeable Np) was assumed to be sorbed on the calcite and apatite moieties.
Sorption in two-mineral systems
Montmorillonite–calcite system
We tested the sorption of Np(V) on three montmorillonite–calcite systems. They contained calcite powder at 1, 3, and 5 wt%, respectively (Hereafter respectively referred to as 1, 3, and 5 % calcite systems). The total sorption on the montmorillonite–calcite system (Fig. 4) was less than that on calcite-free Na-montmorillonite (Fig. 1) and decreased with increasing the content of calcite. Most of the sorbed Np was exchangeable, indicating no evidence of sorption of Np on the calcite moiety. The total sorption of Np on the 3 and 5 % calcite systems were similar to that on Ca-montmorillonite [6]. Calcium and (bi)carbonate ions are released into the liquid phase by the dissolution of calcite. Formation of Np carbonate complexes is negligible below pH 7. Therefore, the reduced sorption on the montmorillonite–calcite systems is due to the interference of the calcium ions released from the calcite moiety. It is known that the sorption of Np(V) on Na-montmorillonite decreases by the addition of Ca2+ ions (as CaCl2) to the liquid phase [6].
Total sorption of Np(V) on the montmorillonite–calcite systems. The data of Ca-montmorillonite were quoted from a previous paper (Kozai et al. [6])
The powder XRD detected the strongest diffraction peak of calcite in the 3 % calcite system but did not detect the diffraction peak in the solid phase after exposure of the 3 % calcite system to 0.01 M NaClO4 aqueous solution, initial pH of 2.1, for 10 days (final pH 4.9) (Fig. 5). The following experiment confirmed that this pH condition was low enough to dissolve calcite completely. When the calcite powder was soaked in a 0.01 M NaClO4 aqueous solution, initial pH 2.1, at the solid-to-liquid ratio of 0.03 g:100 cm3, most of the calcite was dissolved immediately and the residue was completely dissolved in a few days by periodic pH adjustment to 4.9. SEM-EDX analysis of calcite-free Na-montmorillonite did not detect Ca but that for the solid phase after exposure of the 3 % calcite system to aqueous solution at initial pH 2.1 for 10 days did detect Ca (Figure not shown). Probably, the Ca2+ ions released from the calcite moiety partly remained in the liquid phase and the rest was sorbed onto the montmorillonite moiety, and both of those Ca2+ ions interfered with the sorption of Np on the montmorillonite moiety.
Montmorillonite–apatite system
We tested the sorption of Np(V) on two montmorillonite–apatite systems. They contained apatite powder at 2 and 4 wt%, respectively (Hereafter respectively referred to as 2 and 4 % apatite systems).
First, we carried out sorption–desorption experiments for 10 days as a function of pH (Figure not shown). Almost all of the Np was sorbed on the two systems at a final pH above 7, while at a final pH of ca. 3.5, only approximately 20 and 40 % of Np was sorbed on the 2 and the 4 % apatite systems, respectively, and there was a somewhat large variation in the sorption data at that final pH. The variation in the sorption data suggests that the sorption did not reach equilibrium, even after 10 days.
To verify the sorption equilibrium, the time course of the Np sorption on the montmorillonite–apatite systems was investigated at initial pHs of 2.0 and 3.0 (Fig. 6a–c). At the initial pH of 2.0, it took 20 days for the 2 % apatite system and 40 days for the 4 % apatite system to reach sorption equilibrium (Fig. 6a, b). Regarding the 2 % apatite system, the total sorption increased with time until 20 days by the increase of the unexchangeable sorption. The exchangeable sorption was roughly constant over the whole period but varied somewhat widely. The exchangeable sorption at 20 and 40 days was larger than that for apatite-free Na-montmorillonite (Fig. 1). For the 4 % apatite system, the total sorption increased with time until 40 days by the increase of the unexchangeable sorption (Fig. 6b). The exchangeable sorption was more stably constant than that for the 2 % apatite system and less than that for apatite-free Na-montmorillonite (Fig. 1). The sorption–desorption behavior at initial pH 3.0 reached equilibrium in 10 days (Fig. 6c).
The results at initial pH 2.0 are unusual. The sorption of metal ions by a simple mechanism, such as ion exchange and surface complexation, generally reaches equilibrium in a short period. It is also known that the sorption by combined mechanisms (e.g. ion exchange and the subsequent reduction) reaches equilibrium in several days [10, 15]. It could therefore be deduced that a more complex reaction occurred in the experiments at initial pH 2.
The powder XRD detected the strongest diffraction peak of apatite in the 4 % apatite system but did not detect the strongest diffraction peak of apatite in the solid phase after exposure of the 4 % apatite system to a 0.01 M NaClO4 aqueous solution at initial pH 2.0 for 10 days (final pH 3.8) (Fig. 7). The following experiment confirmed that this pH condition was low enough to dissolve the apatite moiety completely. When the apatite powder was soaked in a 0.01 M NaClO4 aqueous solution at initial pH 2.0 and at the solid-to-liquid ratio of 0.04 g:100 cm3, most of the apatite was dissolved immediately, and the residue was completely dissolved in a few days by periodic pH adjustment to 3.8. SEM-EDX analysis detected Ca and P (PO4 3−) on the solid phase after the exposure of the 4 % apatite system to the aqueous solution (Figure not shown). Both Ca and P were non-uniformly distributed at an average of 1.2 wt% (0–2 %) on a metal element basis. Ca was detected with and without P, but P was always detected together with Ca. These detected Ca and P were the ones that were leached from the apatite moiety and then sorbed onto the montmorillonite moiety. The unexchangeable sorption of Np could be the sorption on the montmorillonite moiety mediated by the sorbed Ca2+ and PO4 3− ions.
The unexchangeable sorption of Np that occurred soon after the introduction of the 4 % apatite system to the solution with initial pH 2.0 (the data at 1 days in Fig. 6b) might represent sorption on the yet-to-be-dissolved apatite moiety. However, it is unlikely that the subsequent slow, unexchangeable sorption of Np occurred on the undissolved apatite moiety because the initial apatite residue was supposed to be fully dissolved in the subsequent experiment period. The slow, unexchangeable sorption of Np would be the same as that on the 2 % apatite system.
Figure 8a shows the sorption–desorption profiles of Np(V) for the 2 % apatite systems after the 40 days sorption experiments. At final pH <6, the total sorption of Np increased with increasing pH while the exchangeable sorption decreased with increasing pH. Interestingly, at final pH near 4, more Np was exchangeable (ca. 30 %) on the 2 % apatite system than on apatite-free Na-montmorillonite (ca. 20 %) (Fig. 1). As discussed, under the low pH conditions, the Ca2+ ions leached from the apatite moiety are supposed to have been present in the liquid phase and on the montmorillonite moiety. It is known that Ca2+ ions in liquid phase and on montmorillonite reduce the sorption of Np(V) on montmorillonite [6]. The present results are therefore inconsistent with the previous knowledge. The only difference between the related previous papers and the present study is the presence of phosphate ions leached from the apatite moiety. The phosphate ions might be involved in the unique sorption of Np(V) on the montmorillonite–apatite systems at low pH, i.e., the increased exchangeable sorption (Fig. 8a) and the unexchangeable sorption with slow kinetics (Fig. 6a, b).
At a final pH above 6, almost all of the Np was sorbed on the 2 % apatite system (Fig. 8a). The exchangeable sorption was nearly constant (ca. 10 %) and less than that of the Np sorption on apatite-free Na-montmorillonite (Fig. 1), showing that Np was accumulated on the apatite moiety.
Figure 8b shows the sorption–desorption profiles of Np(V) for the 4 % apatite system after the 40 days sorption experiments. Almost all of the Np was sorbed on the solid phase and approximately 10 % of Np was exchangeable over the tested pH range. The results near neutral final pH also show that Np accumulated on the apatite moiety. The results near final pH 4 do not indicate accumulation on the apatite moiety but rather the accumulation of Np by an unknown unexchangeable sorption, possibly on the montmorillonite moiety.
Phosphate minerals including apatite have high sorption abilities for many heavy elements, and phosphates of heavy elements generally have low solubilities in natural waters [20–22]. Therefore, phosphates are regarded as not only powerful sorbents for heavy elements but also promising engineered barrier materials to prevent migration of heavy elements from contaminated sites to the biosphere [23–25]. A defect of phosphates is that when phosphates are used singly under acidic conditions phosphates lose their high sorption abilities due to their dissolution. However, the finding of this study suggests that the defect of phosphates may disappear in mixed-mineral systems.
Conclusions
This paper studied the sorption behavior of Np(V) on Na-montmorillonite—calcite systems and Na-montmorillonite—apatite systems in the final pH range from 4 to 8 by using the clear difference in the desorption behaviors from those minerals with a 1 M KCl aqueous solution.
Na-montmorillonite showed low sorptivity for Np(V) while calcite and apatite showed high sorptivities for Np(V) when these minerals were used alone. When calcite and apatite were added to Na-montmorillonite, they brought about the opposite effect on the sorption on the montmorillonite-based systems.
The sorption of Np onto the Na-montmorillonite–calcite system (1–5 % calcite) was less than that on calcite-free Na-montmorillonite, due to the interference from the Ca2+ ions leached from the calcite moiety. No evidence of the sorption of Np on the calcite moiety was observed due to the dissolution of calcite.
In the Na-montmorillonite–apatite system (2–4 % apatite), under mild pH conditions at which apatite was hardly dissolved, the sorption of Np on the montmorillonite moiety was less than that on apatite-free Na-montmorillonite, and Np accumulated on the apatite moiety. Under the low pH conditions at which apatite was partly or fully dissolved, the sorption of Np on the montmorillonite moiety was roughly constant while the unknown slow, unexchangeable sorption of Np increased with time. The apatite in this system therefore has effects of accumulating Np regardless of whether the apatite moiety remains undissolved or not.
References
Tochiyama O, Yamazaki H, Mikami T (1996) Sorption of neptunium(V) on various aluminum oxides and hydrous aluminum oxides. Radiochim Acta 73:191–198
Del Nero M, Made B, Bontems G, Clément A (1997) Adsorption of neptunium(V) on hydrargilite. Radiochim Acta 76:219–228
Niitsu Y, Sato S, Ohashi H, Sakamoto Y, Nagao S, Ohnuki T, Muraoka S (1997) Effects of humic acid on the sorption of neptunium(V) on kaolinite. J Nucl Mater 248:328–332
Nakata K, Nagasaki S, Tanaka S, Sakamoto Y, Tanaka T, Ogawa H (2000) Sorption and desorption kinetics of Np(V) on magnetite and hematite. Radiochim Acta 88:453–457
Neck V, Kim JL, Kanellakopulos B (1992) Solubility and hydrolysis behavior of neptunium(V). Radiochim Acta 56:25–30
Kozai N, Ohnuki T, Muraoka S (1995) Sorption behavior of neptunium on bentonite: effect of calcium ion on the sorption. Mater Res Soc Symp Proc 353:1021–1028
Konishi M, Yamamoto K, Yanagi T, Okajima Y (1988) Sorption behavior of cesium, strontium and americium ions on clay materials. J Nucl Sci Technol 25:929–933
Shibutani T, Yui M, Yoshikawa H (1994) Sorption mechanism of Pu, Am and Se on sodium-bentonite. Mater Res Soc Symp Proc 353:725–730
Allard B, Olofsson O, Torstenfelt B, Kipatsi H, Andersson K (1982) Sorption of actinides in well-defined oxidation states on geologic media. In: Lutze VW (ed) Scientific basis for nuclear waste management V. New York, Elsevier, pp 775–782
Kozai N, Ohnuki T, Matsumoto J, Banba T, Ito Y (1996) A study of specific sorption of neptunium(V) on smectite in low pH solution. Radiochim Acta 75:149–158
Ropp R (2013) Encyclopedia of the alkaline earth compounds, 1st edn. Elsevier, New York
Fuller WH (1972) In: Fairbridge WR (ed) The Encyclopedia of geochemistry and environmental sciences, vol IVA. Van Nostrand Reinhold, New York
Klein C, Hurlbut CSJ (1999) Manuel of mineralogy. Wiley, New York
Moore RC, Holt K, Zhao H, Hasan A, Awwad N, Gasser M, Sanchez C (2003) Sorption of Np(V) by synthetic apatite. Radiochim Acta 91:721–727
Zaverin M, Roberts SK, Sawvel AM, Kersting AB (2005) Eu(III), Sm(III), Np(V), and Pu(IV) sorption to calcite. Radiochim Acta 93:93–102
Heberling F, Scheinost AC, Bosbach D (2011) Formation of ternary neptunium(V) bicarbonate inner-sphere sorption complex inhibits calcite growth rate. J. Contam Hydrol 124:50–56
Kozai N, Ohnuki T, Muraoka S (1993) Sorption characteristics of neptunium by sodium-smectite. J Nucl Sci Technol 30:1153–1159
Brown G, Newman ACD, Rayner JH, Weir AH (1978) The structures and chemistry of soil clay minerals. In: Greenland DJ, Hayes MHB (eds) The Chemistry of Soil Constituents. Wiley, Chichester
Thakur P, Moore RC, Choppin GR (2006) Np(V)O2 + sorption on hydroxyapatite-effect of calcium and phosphate anions. Radiochim Acta 94:645–649
Chattanathan SA, Clement TP, Kanel SR, Barnett MO, Chatakondi N (2013) Remediation of uranium-contaminated groundwater by sorption onto hydroxyapatite derived from catfish bones. Water Air Soil Pollut 224:1429. doi:10.1007/s11270-012-1429-5
Oliva J, Pablo JD, Cortina JH, Cama J, Ayora C (2011) Removal of cadmium, copper, nickel, cobalt and mercury from water by Apatite II: column experiments. J Hazard Mater 194:312–323
Lazarević S, Janković-Častvan I, Tanasković D, Pavićević V, Janaćković D, Petrović R (2008) J. Environ Eng-ASCE 134:683–688
Sasaki K, Tsuruyama S, Moriyama S, Handley-Sidhu S, Renshaw JC, Macaskie LE (2012) Ion exchange capacity of Sr2+ onto calcined biological hydroxyapatite and implications for use in permeable reactive barriers. Mater Trans 53:1267–1272
Conca JL, Wright J (2006) An apatite II permeable reactive barrier to remediate groundwater containing Zn, Pb and Cd. Appl Geochem 21:1288–1300
Simona FG, Biermanna V, Segebadeb C, Hedrich M (2004) Behaviour of uranium in hydroxyapatite-bearing permeable reactive barriers: investigation using 237U as a radioindicator. Sci Total Environ 326:249–256
Acknowledgments
Financial support was provided by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (a Grant-in-Aid for Scientific Research(C), No. 23360417).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kozai, N., Yamasaki, S. & Ohnuki, T. Application of simplified desorption method to study on sorption of neptunium(V) on montmorillonite-based mixtures. J Radioanal Nucl Chem 299, 1581–1587 (2014). https://doi.org/10.1007/s10967-013-2896-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2896-x