Abstract
Fallout with rain from the Fukushima reactor accident was monitored for about two months in Higashi-Hiroshima City, Hiroshima, Japan, from March 20 to May 23, 2011. Gamma-ray (γ-ray) spectra measured using a low background HPGe spectrometer showed clear evidence of fission products—131I, 137Cs, and 134Cs. The 131I was observed on March 27 and April 8, while 137Cs and 134Cs were observed on March 27, April 18, and April 22. The 131I, 137Cs, and 134Cs activity concentrations in rainwater collected in Hiroshima reached 0.44 ± 0.09 on April 8, 0.17 ± 0.01 on April 18, and 0.15 ± 0.01 Bq/L on April 18, 2011, respectively. These activity levels were compared with global results collected from the Northern Hemisphere. Several samples of rainwater that were collected in Chiba (Kashiwa) on March 21, April 11, and May 12; Tokyo (Nerima) on March 21 and April 11; Osaka (Hirano) on April 8; Nara (Kitakatsuragi) on April 9; and Fukushima (Fukushima) on April 19, were also measured by our spectrometer and compared. Among these samples of rainwater collected at different locations in Japan, the one collected at Kashiwa City, Chiba Prefecture, on March 21 showed the highest activity concentrations of 6072 ± 1, 632 ± 4, 766 ± 3, 637 ± 1, 97.6 ± 0.8, and 752 ± 2 Bq/L for 131I, 132I, 132Te, 134Cs, 136Cs, and 137Cs, respectively. The health risks due to these high activity levels were considered in terms of several regulations. The ratio of the activites for the isotopes 131I to 137Cs and 134Cs to 137Cs were compared with the other measurements and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The big earthquake (M9.0) at 14:46 p.m. on March 11, 2011 was followed by a big tsunami that caused the severe accident at Fukushima Daiichi Nuclear Power Plant. Due to the blackout, hydrogen detonation occurred at Unit-1 at 15:36 p.m. on March 12, Unit-3 at 11:01 a.m. on March 14, and explosion occurred at Unit-2 at around 6 a.m. on March 15, sequentially, and the estimated amounts of 160, 18, and 15 × 1015 Bq were released for 131I, 134Cs, and 137Cs, respectively [1, 2]. Its spread/distribution of radioactivity was a big concern, and monitoring radioactive gas and airborne particles such as dust and aerosol were reported in several papers [3–9]. However, not much data on fallout with rain [6, 10–13] has been open to the public, compared with that in the air. The importance of direct measurement of fallout with rain is pointed out by the fact that the rise of air dose rate was clearly correlated with rainfall on March 21, measured at Chiba City by the Japan Chemical Analysis Center [14]. Diffusion simulation also showed that radioactive plume was delivered to the Kanto area during this time period [15–17]. On the next day (March 22), radiation levels spiked with the highest number of 210 Bq/L for 131I in Kanto area (Tokyo, Chiba, etc.) tap water [18], and concerns about safety spread since there was more than twice the recommended limit of 100 Bq/L for infants (lower than that of 300 Bq/L for adults). In these respects, we monitored fallout with rain in Hiroshima from March to May 2011, and also checked several sites/dates including the above mentioned Tokyo, Chiba, and Fukushima, Osaka, Nara Prefecture.
Experimental
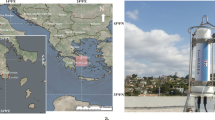
We started sampling rainfall at the Graduate School of Engineering, Hiroshima University, located at Higashi-Hiroshima City (34°40’N, 132°71’E), where the distance is 820.9 km from the Fukushima Daiichi Nuclear Power Plant (37°45’N, 141°27’E), about 40 km east of Hiroshima-City, Japan, from March 20 to May 23, 2011. Samples were collected directly by placing baskets on the roof of a lecture room building during a heavy rainfall (sampling volume > about one liter) and by a large area of roof gutters in case a little rainfall. Rain samples were also collected in several cities at Kashiwa City (Hananoi), Chiba Prefecture (35°90’N, 139°96’E) on March 21, April 11, and May 12; Nerima City (Shakujii), Tokyo (35°73’N, 139°58’E) on March 21 and April 11; Fukushima City (Mikawaminami), Fukushima (37°76’N, 140°46’E) on April 19; Hirano City (Hiranohonmachi), Osaka (34°62’N, 135°55’E) on April 8; and Kitakatsuragi-gun (Koryo Town), Nara (34°55’N, 135°72’E) on April 9, 2011. Distances from the Fukushima Daiichi Plant were 194.7 km, 227.5 km, 63.2 km, 584.2 km, and 575.2 km, respectively (Fig. 1). Collection periods varied from a few tens of minutes to approximately one day.
Following collection, chemical processing of enrichment was done to the rainwater samples in Hiroshima before gamma-ray counting. Rainwater samples varied in volume from 0.030 to 1 liter and were poured into a stainless tray whose surface was covered by a Teflon sheet. First, 2 mL of CsCl (1 mol/L) was added to a co-precipitate for iodine. Second, 2 mL of NaI (0.1 mol/L) was poured to prevent the volatilization of iodine. Third, 0.15 mL of laundry starch was introduced to prevent residue scatter. Then, the sample was evaporated to dryness on an electric hot plate from 125 to 80, and at 50 °C, dried residue was attached on a Teflon sheet. In the end, the Teflon sheet was encapsulated in a polystyrene petri dish (with a diameter of 5.2 cm) for gamma-ray counting.
All the rainwater samples were measured by our low-background, high-purity germanium (HPGe) spectrometer in Higashi-Hiroshima (see Table 1). The spectrometer was a coaxial-type HPGe detector with 12.5 cm of lead, 5cm of steel, 5 mm of copper shield (LBR-2-2, SEIKO EG&G), and 5 mm of acrylic for hermetic shielding to protect against radon in the air, and was located at the Isotope Center at Hiroshima University [20]. It should be noted that this steel was special low-background steel salvaged from the battle ship “Mutsu.” The contamination of 60Co in steel was checked to be 0.03 ± 0.004 (Bg/kg) at the maximum for Mutsu [19] and 0.37 Bq/kg for modern steel [20].
The raw γ-ray energy spectra from 0.05–3.00 MeV were collected for the live time of 80000 sec (about one day) for each sample (See Fig. 2). The detector was calibrated using the natural radioactive background itself. The detection efficiency for γ-rays has been measured with standard sources of known decay rates such as 137Cs, 54Mn, 88Y, 60Co, 109Cd, 139Ce, 51Cr, and 85Sr covering the energy range of 0.08–2.00 MeV and compared with the GEANT4 simulation by taking each geometry into account.
γ-ray spectra of rainwater samples and background measured with HPGe detector. Each measurement time is about one day. Black Kashiwa City, Chiba, data from March 21, 2011. Red Nerima City, Tokyo, data from March 21, 2011. Blue Higashi-Hiroshima City, Hiroshima, data from March 27, 2011. Green Background (no sample)
As examples of high-rate counting data, the energy spectra for Chiba and Tokyo on March 21 were also compared in Fig. 2. These rainwater samples were delivered to our laboratory as per the request of local residents there for its measurements. It was noted that there was a time-lag from 2.9 to 49.2 days depending on the sample, between when the sample was collected and when the sample was measured, due to three unavoidable effects: the sample collection time (10 minutes to about one day); the sample preparation time, such as chemical enriching/distilling rainwater (about a few hours); and the sample counting time (about one day), in addition to trivial human delays such as sample delivering time and waiting time for finishing the other measurements as a result of only one available HPGe spectrometer. Since we needed to know how much of each isotope was present when the sample was collected, the activities had to be corrected for decay back to the time of collection by taking each half-life into account. The correction was obtained by the following formula [21]:
where A 0 was an initial activity defined to be the midpoint of the sample collection time period; A measured was the obtained activity by the energy spectrum; DT = t 2 − t 1 was the measurement time via the beginning of the measurement as t 1; and the end of measurement as t 2, T 1/2 was the half-life of the isotope. Note that one exception was 132I, since 132I is essentially in secular equilibrium with 132Te due to its short half-life (2.3 h). Thus, we used the 132Te half-life to approximate the initial quantity of 132I.
Results
The first rainwater sample was collected on March 20, 2011 in Hiroshima. This sample showed no evidence of fission fragment γ-rays and all visible γ-ray peaks could be attributed to known background sources from the decays of the progeny of 238U and 232Th, and 40K, which did not originate from water, but were part of the natural background present in our laboratory environment including the detector system as impurities. No detection of fission products on March 20 in Hiroshima were matched with the result of no detection measured on the same date in Nagoya [11], which is much closer to the troubled reactors in Fukushima. This may be due to the fact that the radioactive plume was delivered to the Pacific Ocean on that date [15–17]. The first sample that showed fission products was collected on March 27 in Hiroshima, and the samples from subsequent days showed clear peaks attributable to 131I at 364.5 keV, etc.; 134Cs at 604.7 keV, etc.; and 137Cs at 661.7 keV, as seen in Fig. 2. For comparison, background spectrum was measured without a sample, which was shown in Fig. 2. A similar spectrum was also obtained by measuring a 1-liter sample of Higashi-Hiroshima tap water. From this spectrum, we set a 3-sigma upper limit on the 131I, 132I, 134Cs, 136Cs, 137Cs, 132Te, and 7Be activity in this sample to be 0.04, 0.03, 0.03, 0.03, 0.03, 0.03, and 0.27 Bq/liter, respectively.
The sampling dates and measured rainwater activities for the first 73 days of data are listed in Table 2. We cross-checked for consistency between the activities obtained from different branches of the same isotope. As they were in agreement and the systematic error dominates, we only gave the value for the strongest branch. 7Be activity was also compared since 7Be is recognized as an atmospheric tracer due to the fact that its measurement gives information about diffusion properties of the high troposhere and low stratosphere, as well as a description of environmental processes such as precipitation, washout, atmospheric particle deposition, and depositional patterns of airborne contaminants [22].
Several samples of rainwater were collected in Chiba (Kashiwa) on March 21, April 11, and May 12; Tokyo (Nerima) on March 21 and April 11; Osaka (Hirano) on April 8; Nara (Kitakatsuragi) on April 9; and Fukushima (Fukushima) on April 19, and were also checked for their γ radioactivity by our spectrometer and compared. Among these samples of rainwater collected at different locations in Japan, the one collected at Kashiwa City, Chiba Prefecture, on March 21 showed the highest activity concentrations of 6072 ± 1, 632 ± 4, 766 ± 3, 637 ± 1, 97.6 ± 0.8, and 752 ± 2 Bq/L for 131I, 132I, 132Te, 134Cs, 136Cs, and 137Cs, respectively. We believe the activity concentration in Fukushima would have been higher if we collected the rainwater samples in March, not around the middle of April, 2011, and we regret that we were unable to do so.
Using all of our measured data in Table 2, the ratio of activities of 131I and 134Cs to 137Cs were calculated and plotted against the time since the earthquake (days) in Fig 3. The weighted average ratio of 134Cs to 137Cs was found to be 0.92 ± 0.03 in agreement with the value of 0.8 – 0.9, reported in the whole investigated region, including Fukushima Prefecture [23]. This equally produced 134Cs will be an indication of the nuclear reactor accident rather than a nuclear weapon test since the formation of 134Cs via neutron capture on 133Cs requires a length of criticality. Even though iodine and cesium have different chemical properties, the ratio of 131I to 137Cs can be used to obtain information about when the nuclear reactions terminated. This iodine–cesium dating was applied to the data of the Tokyo Electric Power Company (TEPCO) for seawater samples at several monitoring posts near the troubled reactor and a water sample taken from the spent-fuel cooling pool of the Unit-4 reactor building by Matsui [24]. The same exponential function was applied to our current data:
where f I and f Cs are the fractions of 131I and 137Cs per fission (2.89 × 10−2 and 6.19 × 10−2, respectively; [25] τI and τCs are the lifetimes of 131I and 137Cs; and \(\Updelta t\) is the length of time the reactor has been active. The best fit value for \(\Updelta t\) was found to be 1.5 years with large deviations (χ2/dof ≥ 5), but are in good agreement with the one year reported by Matsui.
Ratio of activities for the isotopes 131I to 137Cs (black) and 134Cs to 137Cs (red). The error bar shows the statistical error only as the systematic errors cancel to a large degree in the ratios. The fits to the ratios as a function of time were performed with fixed decay time constants (dashed line). The weighted average ratio of 134Cs to 137Cs was found to be 0.92 ± 0.03, which was plotted as a black line
Discussion
The 131I was observed only at the early dates on March 27 and April 8, while 137Cs and 134Cs were observed on March 27, April 18, and April 22 in Hiroshima. This means that the radioactive cloud was first richer in 131I, as iodine is a more volatile element than cesium. The 131I, 137Cs, and 134Cs activity concentrations in rainwater collected in Hiroshima reached 0.44 ± 0.09 on April 8, 0.17 ± 0.01 on April 18, and 0.15 ± 0.01 Bq/L on April 18, 2011, respectively. The highest activity level in Hiroshima was compared with global results (Table 3), which were collected in the Northern Hemisphere. Among these results on continuous surveys, except for occasional spot checks such as the results in Chiba, Tokyo; Fukushima, Osaka; and Nara, the highest values for all radioiodine and radiocesium were measured at Berkeley, Oakland, and Albany, California, USA. Note that all the dates of the highest activities reported in Western countries were earlier than that of western Japan (Hiroshima and Nagoya). This will be affected by specific local weather conditions on these dates, and will undoubtedly be a complicated function of many variables including release rates at the Fukushima reactor site and wind patterns and velocities. These may be attributed to the general trend of typical winter pressure patterns in Japan, such as the high-pressure area to the west and low-pressure area to the east, and the Westerlies (jet stream flowing west to east), which are strongest in the winter hemisphere.
This maximum activity can be compared to the regulation set by the Ministry of Health, Labour, and Welfare, Japan, on 131I and 137Cs allowed in drinking water of 300 Bq/L (100 Bq/L for infants) and 200 Bq/L, respectively Footnote 1. The activity collected at Nerima, Tokyo, on March 21 of 614 ± 1 for 131I was also beyond this regulation and coincided with the situation that the activity of tap water at the water purification plant in Tokyo and Chiba exceeded this regulation on March 22. The maximum level of 131I collected at Kashiwa city, Chiba, exceeded the IAEA Operational Intervention level for nuclear emergencies at 3000 Bq/L, too. Except for the two cases in Chiba and Tokyo on March 21, all the other results were below the regulation. It is noted that the observed reduction factors of about 10−5 and 10−4 after 52.6 days from March 21 to May 12 for its activity of 131I and 137Cs, respectively, are much larger than the expected decrease of about 10−2 and 0.997 due to the half-life of 8.0 days and 30.1 years, respectively. This is attributable to the fact that the activity largely depends on its transport/deposition with rain. If a person were to drink two liters of water per day containing the highest activity level of 131I in Chiba on March 21, then in one year he or she would receive a whole body dose of about 70 mSv, based on the procedure set by the MEXT [26]. This can be compared with the ICRP (NRC) limits that no worker exceeds 50 mSv to the whole body or 500 mSv to the thyroid, and that no member of the public exceeds 1 mSv to the whole body, but as described in the previous sentence, this is not the case.
Note that no fission products were detected in Nara, even though the same date and the distance between Osaka and Nara is only about 9 km. It has been suggested that western Japan was sheltered by mountain ranges from nonvolatile elements such as Cs, which was absorbed into aerosols [23, 27]. A similar condition may have occurred in Nara, i.e., the transport of particulate material and radioactive plume may have been blocked by the Ikoma Mountain (642 m height) between Osaka and Nara, which forms the Nara basin.
Conclusion
Fallout with rain from the Fukushima reactor accident was monitored for about two months from March 20 to May 23, 2011 in Higashi-Hiroshima City, Hiroshima. The activities of nuclear fission products reached 0.44 ± 0.09 Bq/L on April 8 and 0.17 ± 0.01, 0.15 ± 0.01 on April 18 for 131I, 137Cs, and 134Cs, respectively. Our result was compared with other reported data from the Northern Hemisphere, and these highest activities and their detected dates in rainwater samples will help to understand the release of radioactive fission products and their transport in the atmosphere. Several rainwater samples collected at Chiba (Kashiwa), Tokyo (Nerina), Osaka, Nara, and Fukushima were also checked. Chiba data showed the highest activities of 6072 ± 1, 752 ± 2, 637 ± 1 Bq/L on March 21, 2011 for 131I, 137Cs, and 134Cs, respectively. The activity collected at Nerima, Tokyo, on March 21, 2011 of 614 ± 1 for 131I, was also beyond the regulation. Except for the two cases in Chiba and Tokyo on March 21, 2011, all the other results were below the regulation. Fortunately, the observed reduction of these activities after about 50 days was much larger than the expected decrease due to its decay-out. However, regular monitoring would be advised. The ratio of the activities for the isotopes 131I to 137Cs and 134Cs to 137Cs were also checked and found to be consistent with the other measurements.
Notes
Since April 2012, the Ministry has set this regulation back to being 10 Bq/L, which had been applied until March 16, 2011.
References
Ohara T, Morino Y, Tanaka A (2011) Atmospheric behavior of radioactive materials from Fukushima Daiichi Nuclear Power Plant. J Natl Inst Public Health 60(4):292 (in Japanese)
Brumfiel G (2011) Fallout forensics hike radiation toll. Nature 478:435
Bowyer TW et al (2011) Elevated radioxenon detected remotely following the Fukushima nuclear accident. J Environ Radioact 102:681
Sinclair LE et al (2011) Aerial measurement of radioxenon concentration off the west coast of Vancouver Island following the Fukushima reactor accident. J Environ Radioact 102:1018
Diaz Leon J et al (2011) Arrival time and magnitude of airborne fission products from the Fukushima, Japan, reactor incident as measured in Seattle, WA, USA. J Environ Radioact 102:1032
Manolopoulou M et al (2011) Radioiodine and radiocesium in Thessaloniki, Northern Greece due to the Fukushima nuclear accident. J Environ Radioact 102: 796; Radiation measurements and radioecological aspects of fallout from the Fukushima nuclear accident. J Radioanal Nucl Chem. doi:10.1007/s10967-011-1386-2
Fushimi K et al (2011) Measurement of airborne radioactivity from the Fukushima reactor accident in Tokushima, Japan. arXiv: 1104.3611[nucl-ex]
Shizuma K (2011) Survey of radioactivity concentration in the Kagamiyama area’s atmosphere, Hiroshima University. http://www.hiroshima-u.ac.jp/en/top/news_events/earthquake/sokuteikekka/
MacMullin S et al (2012) Measurement of airborne fission products in Chapel Hill, NC, USA from the Fukushima Dai-ichi reactor accident. J Environ Radioact 112:165
Norman E et al (2011) Observations of fallout from the Fukushima reactor accident in San Francisco Bay area rainwater. arXiv: 1103.5954[nucl-ex]
Ogata Y (2012) Fallout from Fukushima accident in Nagoya. In: Proceedings of the seminar at Nagoya by radiation education forum, January, 5(in Japanese): radioiodine and radiocesium in fallout with rain in Nagoya from March to May 2011, proceedings of the annual meeting of Japan health physics society, October, 102 (2011) (in Japanese): the 7th international workshop on ionizing radiation monitoring, December (2011), to be published in Radiation Measurements
Pittauerova D, Hettwig B, Fischer HW (2011) Fukushima fallout in Northwest German environmental media. J Environ Radioact 102:877
Cleveland BT et al. (2012) Activities of γ-ray emitting isotopes in rainwater from Greater Sudbury, Canada, following the Fukushima incident. Can J Phys 90:599
Japan Chemical Analysis Center. http://www.jcac.or.jp/senryoritu_kekka.html
Japan Atomic Energy Agency, WSPEEDI. http://www.jaea.go.jp/jishin/kaisetsu03/kaisetsu03.htm
Central Institute for Meteorology and Geodynamics, Austria. http://www.zamg.ac.at/
Deutscher Wetterdienst (DWD), Germany. http://www.dwd.de/
ex. CBSNEWS. Radiation levels spike in Tokyo tap water. http://www.cbsnews.com/stories/2011/03/23/501364/main20046120.shtml
Matsuoka K, Ejiri H (1989) Low-level radioactive isotopes contained in materials used for beta-ray and gamma-ray detectors. OULNS Ann Rep 54
Shizuma K, Yokohata K, Inada K (2004) Background reduction of low level gamma-ray spectrometer. Bull Grad School Eng Hiroshima Univ 53:87
UC Berkeley Nuclear Engineering Air Monitoring Station. http://www.nuc.berkeley.edu/UCBAirSampling
Megumi K et al (2000) Factors, especially sunspot number, causing variations in surface air concentrations and depositions of 7Be in Osaka, Japan. Geophys Res Lett 27:361
Kinoshita N et al (2011) Assessment of individual radionuclide distributions from Fukushima nuclear accident covering central-east Japan. Proc Nat Acad Sci 108:19526
Matsui T (2011) Deciphering the Measured Ratios of Iodine-131 to Cesium-137 at the Fukushima Reactors. Prog Theor Phys 126:1167
Isotopes Project, Fission Product Yields: http://ie.lbl.gov/fission.html
Dose conversion coefficient of 1.63 × 10−8 Sv/Bq has been applied: http://www.mext.go.jp/component/b_menu/shingi/giji/_icsFiles/afieldfile/2012/01/13/1315036_5.pdf
Yasunari TJ et al (2011) Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc Nat Acad Sci 108:19530
Acknowledgements
The author (R. H.) thanks Prof. S. Nakashima, Prof. K. Shizuma and all the staff at the Isotope Center at Hiroshima University for their kind assistance. We also gratefully acknowledge discussions with Dr. Y. Ogata (Nagoya University). The author is grateful to H. Tazaki, T. Yatsuo, E. Hazama, and M. Hazama for providing the samples. The work of R. H. is supported by KAKENHI (the Grant-in-Aid for Scientific Research C: 23561013) by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hazama, R., Matsushima, A. Measurement of fallout with rain in Hiroshima and several sites in Japan from the Fukushima reactor accident. J Radioanal Nucl Chem 297, 469–475 (2013). https://doi.org/10.1007/s10967-012-2417-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2417-3