Abstract
1-(2-methoxy phenyl) piperazine fragment of WAY100635 or its phenolic analogue, derived from DWAY is used to design the desired structure of 5HT1A receptor imaging agents. In this study a DWAY analogue was labeled with 99mTc-nitrido ([99mTcN]2+) core via dithiocarbamate. 2-(piperazin-1-yl) phenol dithiocarbamate was synthesized by the reaction of 2-(piperazin-1-yl) phenol with an equivalent amount of carbon disulfide in KOH solution then radiolabeled with 99mTc-nitrido core. The final complex was characterized by HPLC and its radiochemical purity was more than 90 %. In vitro stability studies have shown the complex was stable at least 4 h after labeling at room temperature. The n-octanol/water partition coefficient experiment demonstrated log p = 1.34 for 99mTcN–OHPP–DTC. Biodistribution results showed that radio tracer had moderate brain uptake (0.39 ± 0.03 %ID/g at 15 min and 0.29 ± 0.02 %ID/g at 120 min) and good retention, suggesting that this complex may lead to a further development of a radiotracer with specific binding to 5-HT1A receptor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The 5HT1A subtype of receptors for the neurotransmitter serotonin is involved in important physiological processes in the human brain, and changes in their density cause several neurological disease, such as depression, schizophrenia and Alzheimer [1]. New radioligands as a diagnostic tool for evaluation of neuroreceptors in the central nervous system (CNS), either by positron emission tomography (PET) or single-photon emission computed tomography (SPECT) were attracted in the past years [2]. Recently many compounds as PET radiotracers for imaging of 5HT1A receptor have been studied [3–12]. Despite the great variety of 5HT1A radioligands with PET, there is still a scope for the development of technetium labeled 5HT1A ligands for SPECT imaging due to the availability of low cost 99Mo/99mTc generator, favorable physical characteristics of 99mTc (t 1/2 of 6 h, γ 140 keV 89 % abundance) and high specific activity of radionuclide [1, 2]. The (2-methoxyphenyl)piperazine (MPP), residue of WAY 100635, which is known to have high affinity to the 5-HT1A receptor, was selected as the ideal structure for the design of potential radiotracers [8, 11]. Many 99mTc labeled complexes carrying MPP moiety with high affinity for the 5HT1A receptor have been reported [13–17]. However, most of those agents challenge with some problems such as low initial brain accumulation and high non-specific uptake. The preparation of a novel 99mTc labeled MPP analogue is still considered to be necessary and highly interesting.
The [99mTcN]2+ is a novel technetium cores, which forms 99mTc-nitrido complexes with high stability as compared to those obtained using the conventional [99mTcO]3+ core. The [99mTcN]2+ core where 99mTc is in +5 oxidation state is very stable, can be prepared as a precursor, and forms stable complexes, therefore acting as a variable alternative to the isoelectronic [99mTcO]3+ core [18]. Additionally, it has high affinity to form complex with ligands containing donor atoms such as sulfur, as found in dithiocarbamates. The 99mTcN–dithiocarbamate complexes formed are neutral and lipophilic, which are necessary criteria for potential brain perfusion imaging agents in facilitating crossing of the blood–brain barrier [19, 20]. Until now a number of 99mTc-nitrido tracers with dithiocarbamate ligands for heart, brain and tumor imaging have been reported [19, 21, 22]. There is much interest to design new 99mTcN-dithiocarbamate ligands to develop better diagnostic agents.
Among 5HT1A receptor ligands, [11C] DWAY the desmethylated analogue of [11C] WAY-100635 [23] showed a significantly higher radioactivity signal [24]. Based on past studies, Defraiteur et al. [25] developed a desmethyl analogue of [18F]p-MPPF: [18F]p-DMPPF, which showed a better brain penetration than that of [18F]p-MPPF. Based on the above mentioned article, in this work the 2-(piperazin-1-yl)phenol (DWAY fragment) was chosen and conjugated to the technetium chelate dithiocarbamate unit. The synthesis of 2-(piperazin-1-yl)phenol dithiocarbamate performed and the labeling of ligand by [99mTcN]2+ core was carried out. This compound in vitro and in vivo stability, partition coefficient, and biodistribution were investigated.
Experimental
Materials and methods
The 2-(piperazin-1-yl) phenol (2PP), Succinic dihydrazide (SDH), propylene diamine tetraacetic acid (PDTA) and stannous chloride dehydrate were purchased from Aldrich chemical company. Potassium 4-(2-hydroyphenyl) piperazine-1-carbodithioate compound was prepared by reaction of 2PP with carbon disulfide in the presence of KOH. All commercially available reagents were reagent grade and used without further purification. Technetium-99m as sodium pertechnetate (Na99mTcO4) was obtained from an in-house 99Mo/99mTc column generator using 0.9 % saline. Fourier transform infrared (FT-IR) spectra was recorded in a KBr pellet in the range 4,000–400 cm−1 on Perkin Elmer spectrometer. High resolution fast bombardment mass spectroscopy was performed using an Agilent 1100/Bruker Daltonic (Ion trap) VL instrument. Monitoring of all reactions was performed with analytical reverse-phase high performance liquid chromatography (RP-HPLC) on a JASCO 880-PU intelligent pump HPLC system (Tokyo, Japan) equipped with a multiwavelength detector and a flow-through Raytest-Gabi g-detector. CC 250/4.6 Nucleosil 120-5 C-18 column from Teknokroma was used for HPLC. Radioactivity measurements were carried out using Na(Tl) scintillation counter (ORTEC Model 4001 M Minibin & Power Supply).
Preparation of 2-(piperazine-1-yl) phenol dithiocarbamate
The potassium salt of 2-(piperazine-1-yl) phenol dithiocarbamate was synthesized by the reaction of 2-(piperazine-1-yl) phenol with an equivalent amount of carbon disulfide in KOH solution. A solution of potassium hydroxide (100 mg, 1.78 mmol) was added to 2-(piperazin-1-yl) phenol (205 mg, 1.15 mmol) in about 7 ml ethanol in the ice bath and stirred then carbon disulfide (200 μl) was added dropwise to this solution. The mixture stirred for an hour in ice bath below 5 °C. A dark precipitate slowly was appeared. The solvent was removed by evaporation under reduced pressure, and the precipitate then washed by ether. The product was recrystallized by using ethanol/diethyl ether to give a semi white crystals of 2-(piperazine-1-yl) phenol dithiocarbamate. FT-IR and Mass analysis methods were used to confirm the structure of the desired complex.
Preparation of 99mTc-nitrido
A vial containing 5.0 mg of SDH, 100 μg of stannous chloride dehydrate, 5.0 mg of PDTA, 0.5 mg of sodium dihydrogen phosphate and 5.8 mg of disodium hydrogen phosphate was used for preparing the nitride intermediate. Then the 1 ml of saline containing 99mTcO4 − (20 mCi, 740 MBq) was added. The vial was then vortexed for 1 min and allowed to stand at room temperature for 20 min.
Radiolabeling of dithiocarbamate ligand with 99mTc-nitrido
For preparation of 99mTc-nitrido-ligand, 1 mg of the potassium salt of 2-(piperazin-1-yl)phenol dithiocarbamate dissolved in 0.5 ml of phosphate buffer (pH 7) in a vial. Then, 500 μl (10 mCi, 370 MBq) of a freshly prepared 99mTc-nitrido was added, vortexed for 1 min and the reaction vial incubated for 15 min at room temperature.
Radiochemical analysis
Radiochemical purity of the 99mTc-nitrido and 99mTc-nitrido-ligand were analyzed by TLC and HPLC. For the characterization of 99mTc-nitrido, TLC plates were developed in ethanol:chloroform:toluene:0.5 M ammonium acetate (6:3:3:0.5 v/v) as well as in saline. In the first solvent, free 99mTcO4 − have a R f = 0.5 while [99mTcN]2+ remain at the point of application. In the second solvent, [99mTcN]2+ move with solvent front with R f = 1 and the reduced technetium remain at the point of application. The radioactivity was quantified by cutting the strip (1.5 × 10 cm2) into 1 cm pieces and counting in a well type gamma counter.
For radiochemical analysis of 99mTc-nitrido and the 99mTc-nitrido-ligand by HPLC a volume of 10 μl of the test solutions were injected into the C-18 reverse phase column and 0.1 % trifluoroacetic acid/water (Solvent A) and acetonitrile (Solvent B) were used as a mobile phase in the following gradient: 0 min 95 % A (5 % B), 5 min 95 % A (5 % B), 25 min 0 % A (100 % B), 30 min 0 % A (100 % B), flow = 1 ml/min, λ = 280 nm.
Octanol/water partition coefficient (log p value)
The octanol/water partition coefficient of complex was measured following 1 min vigorous vortex mixing of 1 ml of octanol and 0.9 ml saline (pH 7), with approximately 100 μl of radiotracers in a micro centrifuge tube. The tubes were centrifuged at 500 rpm for 5 min and the counts in 100 μl aliquots of both organic and inorganic layers were determined by use of a NaI well-type γ-counter. The partition coefficient (P) was calculated using the following equation: P = (cpm in octanol − cpm in background)/(cpm in water − cpm background). The reported octanol/water partition coefficient represents the mean (±standard deviation) of the three measurements.
In vitro stability
The stability of the complex was evaluated by monitoring the radiochemical purity at different time points (15 min, 1, 3 and 6 h) using the following procedures. In a propylene test tube an aliquot of complex was incubated at room temperature (25 °C) for different time periods. The radiochemical purity was determined for each time period by HPLC analysis.
Biodistribution
The in vivo biodistribution study of 99mTc-nitrido-ligand was carried out according to the relevant national regulations using male Wistar rats (5–6 week old). Complex (about 7.4 MBq in 100 μl solution) after HPLC purification was injected through the tail vein. The rats were sacrificed at different post injection times (2–120 min) and the tissues and organs of interest were collected, wet weighed and counted in a NaI well-type γ-counter. The percentage of injected dose per gram (%ID/g) for each sample was calculated by comparing its activity with appropriate standard of injected dose (ID). The values are expressed as mean ± SD. The blocking studies were also done for two complexes by using of 8-OH-DPAT, the putative 5HT1A blocker. Rats received a tail vein injection of a solution (50 μl) of 8-OH-DPAT (2 mg/kg) 1 min before administration of the radiotracer and dissected 30 min after injection.
Results and discussion
Chemistry and radiolabeling
Dithiocarbamate derivative of DWAY was required for subsequent complexation with the 99mTc-core. The amino group of DWAY was simply derivatized to dithiocarbamate using carbon disulfide in the presence of KOH. The reaction was monitored by HPLC and the reaction product was recrystallized and the yield was 58 %. This compound, (OHPP–DTC) was characterized by FT-IR and ESI–MS. FT-IR (KBr)/cm−1: 3,408 ν(O–H), 2,998 ν(C–H), 1,049 ν(C=S). ESI–MS (m/z, percent abundance): 291 ([M + K]+), (100 %).
To prepare the technetium-99m complex of dithiocarbamate, 99mTc-nitrido core was prepared beforehand. The method is based on the reaction of 99mTcO4 − with SDH. This compound acts as an efficient donor of nitride nitrogen atoms (N3−) in the presence of stannous chloride as a reducing agent to form a 99mTc-nitrido intermediate. In order to prevent precipitation of stannous ion in the form of insoluble tin salts the PDTA was added. The [99mTcN]2+ now is a suitable substrate for the substitution reaction with the sodium potassium salt of dithiocarbamate-DWAY at room temperature to give the final complex (Fig. 1). Optimization studies for acquiring maximum complexation yield showed that the radiolabeling yield depends on the reaction pH. The reaction was favorable at acidic pH 5 leading to >90 % complexation at specific activity of 0.1 GBq/μmol. Increasing the pH led to a fall in the complexation yield which decreased to 50 and 10 % at pH 7 and 10, respectively.
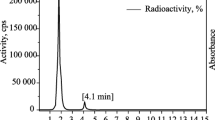
In the radiochemical purity determination of 99mTc-nitrido by TLC, in solvent system ethanol:chloroform:toluene:0.5 M ammonium acetate (6:3:3:0.5 v/v) <3 % of total activity was moved and counted in R f 0.5 belonged to 99mTcO4 −. In saline as a solvent, only minimal activity (<1 %) remained in origin corresponding to reduced technetium-99m. In HPLC analysis 99mTc-nitrido showed a retention time of 3.27 min with more than 95 % yield which was in accordance with its TLC results (Fig. 2). 99mTc-nitrido-ligand HPLC studies demonstrated that the reaction was leaded to a single complex and its retention time was found to be 19.67 min with a yield of more than 90 % (Fig. 3).
Based on the information about synthesis of technetium-nitrido dimmer compounds [26, 27] and also earlier description of the molecular structure of [99mTcN(noet)2](noet = N-ethyl-N-ethoxydithiocarbamato) [28], it appears rational to assume that the structure of 99mTcN–OHPP–DTC is similar to that of 99mTcN(noet)2. The complex have a square pyramidal structure with 99mTc≡N bond in apical while four sulfur atoms from two dithiocarbamate ligands cover four remaining positions in the base surface. To determine exact structure of the complex further research is necessary.
Lipophilicity and stability study
The partition coefficient of the radiolabeled complex was determined by distribution in octanol and water, and the lipophilicity (log p) of 99mTcN–OHPP–DTC was found to be 1.34. The log p of the complex was within the range of values quoted for lipophilicity (0.5–2.5) which is suitable for crossing the blood brain barrier. The radiochemical purity of the 99mTc-nitrido-ligand was nearly constant (>90 %) over the observed period of 6 h. No decomposition of the complex was observed in this time period, suggesting its high stability in the reaction mixtures at room temperature.
Biodistribution study
Biological evaluation of 99mTcN–OHPP–DTC complex was performed in male Wistar rats. The results are shown in Table 1. 99mTc-nitrido-ligand showed significant liver accumulation within 2 min with slow clearance (17.01 ± 1.41 %ID/g at 2 min and 13.86 ± 1.44 %ID/g at 120 min post injection), suggesting the hepatobiliary system is the major route of excretion of the administered radioactivity. As the log p of the 99mTc-nitrido-ligand shows, this manner of acting is probably due to more lipophilicity of this radio tracer. This complex had moderate brain uptake (0.37 ± 0.01 %ID/g at 2 min and 0.29 ± 0.02 %ID/g at 120 min post injection) and good retention. Initial brain uptake and retention may be is related to lipophilicity and in vivo stability of the complex, although the blood uptake was not so high at the beginning (2.99 ± 0.09 %ID/g) and almost 70 % of the radioactivity was cleared out after 120 min post injection (0.90 ± 0.15 %ID/g).
The distribution of the activity in different regions of brain was also performed in Wistar rats (Table 1). For complex 99mTcN–OHPP–DTC the radioactivity concentration of hippocampus (Hipp) at 2 min p.i was 0.41 ± 0.01 %ID/g which was increased to 0.49 ± 0.07 %ID/g at 30 min post injection and about 80 % of its activity was retained in Hipp at 120 min p.i. This is may be due to the clearance of non specific uptake from brain after 60 min post injection, and on the other hand this high retention shows 99mTcN–OHPP–DTC have specific affinity to 5HT1A receptor in the brain. For cerebellum (CB) the uptake was lower than Hipp. The ratio of Hipp/CB was 2.15 at 2 min and 3.16 at 120 min post injection; it shows the accumulation of radioactivity in Hipp (the area of 5HT1A receptors).
Biodistribution of these complexes with blocking agent in different part of brain also was investigated at 30 min post injection (Table 1). The uptake of Hipp was decreased obviously for 99mTcN–OHPP–DTC from 0.49 ± 0.07 %ID/g to 0.25 ± 0.04 %ID/g and the uptake of cortex was also decreased from 0.31 ± 0.04 %ID/g to 0.17 ± 0.02 %ID/g at 30 min post injection but activity in the cerebellum showed no significant decrease. The uptake in hippocampus and cortex was specific and receptor mediated, as shown by the co-injection of blocking agent, indicating that these brain regions are also 5HT1A receptor positive.
In the past few years, 11C labeled WAY-100635 has been developed and successfully used as PET radiotracer [11]. However Studies of the metabolic pathway of [carbonyl-11C]WAY-100635 in human revealed rapid metabolism to WAY-100634 due to the hydrolysis of the amide bond, which interfere the PET measurements [7–9]. In addition, a disadvantage of using carbon-11 (t 1/2 = 20 min) radiotracers is that these tracers can only be used where both a cyclotron and a PET-camera are in close proximity due to it short half-life. Zhuang and co-workers have shown in a series of benzamido analogs of WAY-100635 a limited tolerance for variations at the amino pyridine position in the native receptor [10]. Therefore, research has been directed toward WAY-100635 analogs that were labeled with the longer lived fluorine-18 (t 1/2 = 110 min) isotope with the label outside the WAY-100634 moiety [29] but in addition to the hydrolysis of the amide bond, defluorination was observed [5]. Flowed by successful development of 99mTc-TRODAT [30, 31], fragments of WAY100635 were selected for developing 99mTc-labeled 5HT1A receptor imaging agents [14–16, 31]. As until now there has no ideal 99mTc agent for imaging 5HT1A receptors. Further design of these kind 99mTc complexes could improve the brain uptake with a high receptor affinity. Based on pharmacokinetic behavior and brain distribution results for our radioconjugate, further ligand modification such as using carbon chain spacer could be considered for improvement of this novel 99mTc-complex as a brain receptor imaging agent.
Conclusion
In this study, we have shown design and synthesis of new 5HT1A receptor imaging agent 99mTcN–OHPP–DTC with high radiolabeling yield. According to the results of in vivo biodistribution studies, we found that this complex had moderate brain uptake and a good retention time with more favorable properties for further study. Regional brain distribution study showed a clear correlation between distribution of radioactivity and distribution of 5HT1A receptors in the brain. Blocking studies confirmed that 99mTcN–OHPP–DTC had specific binding to the 5HT1A receptors. In this area further studies regarding the structure modification should be considered to increase regional brain uptake of this 99mTc complex.
References
Passchier J, Waarde AV (2001) Eur J Nucl Med 28:113
Halldin C, Gulyas B, Langer O, Fared Q (2001) J Nucl Med 45:139
Aznavour N, Zimmer L (2007) Neuropharmacology 52:695
Farde L, Ito H, Swahn CG, Pike VW, Halldin C (1998) J Nucl Med 39:1965
Lang L, Jagoda E, Schmall B, Vuong BK, Adams HR, Nelson DL, Carson RE, Eckelman WC (1999) J Med Chem 42:1576
Garcia R, Xavier C, Paulo A, Santos I, Kniess T, Bergmann R, Wust F (2005) J Label Compd Radiopharm 48:301
Le-Bars D, Lemaire C, Ginovart N, Plenevaux A, Aerts J, Brihaye C, Hassoun W, Leviel V, Mekhsian P, Weissmann D, Pujol JF, Luxen A, Comar D (1998) Nucl Med Biol 25:343
Pike VW, Halldin C, Wikstrom H, Marchais S, McCarron JA, Sandell J, Nowicki B, Swahn CG, Osman S, Hume SP (2000) Nucl Med Biol 27:449
Sanabria-Bohorquez SM, Biver F, Damhaut P, Wikler D, Veraart C, Goldman S (2002) Eur J Nucl Med 29:76
Vandecapelle M, Dumont F, Vos FD, Strijckmans K, Leysen D, Audenaert K, Dierckx R, Slegers G (2004) J Label Compd Radiopharm 47:531
Parsey RV, Belanger MJ, Sullivan GM, Simpson NR, Stabin MG, Van Heertum R, Mann JJ (2005) J Nucl Med 46:614
Tipre DN, Zoghbi SS, Liow JS, Green MV, Seidel J, Jchise M, Innis RB, Pike VW (2006) J Nucl Med 47:345
Papagiannopoulou D, Pirmettis I, Tsoukalas C, Nikoladou L, Dros-sopoulou G, Dalla C, Pelecanou M, Papadopoulou-Daifotis Z, Papa-dopoulos M, Chiotellis E (2002) Nucl Med Biol 29:825
Heimbold I, Drews A, Syhre R, Kretzschmar M, Pietzsch HJ, Johannsen B (2002) Eur J Nucl Med 29:82
Heimbold I, Drews A, Kretzschmar M, Varnas K, Hall H, Halldin C, Syhre R, Kraus W, Pietzsch HJ, Seifert S (2002) Nucl Med Biol 29:375
Leon A, Rey A, Mallo L, Pirmettis I, Papadopoulos M, Leon E, Pa-gano M, Manta E, Incerti M, Raptopoulou C (2002) Nucl Med Biol 29:217
Bolzati C, Mahmood A, Malago E, Uccelli L, Boschi A, Jones AG, Refosco F, Duatti A, Tisato F (2003) Bioconjug Chem 14:1231
Baldas J, Bonnyman J (1985) Int J Appl radiat Isot 35:133
Pasqualini R, Duatti A, Bellande E, Comazzi V, Brucato V, Hoffschir D, Fagret D, Comet M (1994) J Nucl Med 35:334
Dischino D, In Nunn AD (1992) Radiopharmaceuticals: chemistry and pharmacology. Marcel Decker Inc, New York, pp 1–35
Zhang JB, Wang XB, Liu J (2005) Appl Radiat Isot 62:33
Chu TW, Li RJ, Hu SW, Wang Y, Liu XQ, Wang XY (2004) J Radioanal Nucl Chem 261:199
Pike VW, Halldin C, McCarron JA, Lundkvisit C, Hiani E, Olsson H, Hume SP, Karlsson P, Osman S, Swahn CG, Hall H, Wikstrom H, Mensonidas M, Poole KG, Farde L (1998) Eur J Nucl Med Mol Imaging 25:338
Andree B, Halldin C, Pike VW, Gunn RN, Olsson H, Farde L (2002) J Nucl Med 43:292
Defraiteur C, Lemaire C, Luxen A, Plenevaux A (2006) Nucl Med Biol 33:667
Baldas J, Boas JF, Colmanet SF, Ivanov Z, Williams GA (1993) Radiochim Acta 63:111
Nicholson T, Kramer DJ, Davison A, Jones AG (2001) Inorg Chim Acta 316:110
Pasqualini R, Duatti A (1992) J Chem Soc Chem Commun 18:1354
Cliffe lA (2000) Nucl Med Biol 27:441
Kung HF, Kim HJ, Kung MP, Meegalla SK, Plossl K, Lee HK (1996) Eur J Nucl Med Mol Imaging 23:1527
Kung HF (2001) Nucl Med Biol 28:505
Acknowledgments
The authors wish to thank Mr. Mirfallah and Mr. Talebi of the radioisotope department (AEOI) for providing sodium pertechnetate and assistance in quality control tests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erfani, M., Hassanzadeh, L., Ebrahimi, S.E.S. et al. 99mTc-labeling of a dithiocarbamate-DWAY fragment using [99mTcN]2+ core for the preparation of potential 5HT1A receptor imaging agents. J Radioanal Nucl Chem 295, 1783–1788 (2013). https://doi.org/10.1007/s10967-012-2298-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2298-5