Abstract
The determination of Zn in geological samples using instrumental neutron activation analysis is usually done using the 64Zn(n,γ)65Zn reaction and its 244 day half-life. However this analysis has proven to be potentially difficult. This is due to its relatively low neutron absorption cross section and gamma ray intensity, and the relatively high neutron absorption cross section and gamma intensity of 46Sc, which has an energy peak that is only 5 keV greater than 65Zn. The use of a high resolution detector makes it possible to differentiate between the 65Zn and 46Sc photopeaks peaks. However, the dominating 46Sc gamma ray can even make peak fitting routines unsuccessful in the proper determination of 65Zn. The use of a Compton suppression system suppresses the 46Sc peak, which has two coincident gamma-rays, and this greatly improves the ratio of the height of the 46Sc 1120.5 keV photopeak to the 65Zn 1115.4 keV photopeak. Irradiating the sample with epithermal neutrons also improves the measurement since 65Zn has a higher cross section for epithermal neutrons rather than thermal neutrons, whereas 46Sc has a higher thermal cross section. Another technique to determine zinc is the use of 68Zn(n,γ)69mZn reaction with its 13 h half-life using epithermal neutrons and Compton suppression INAA. However, the 438 keV gamma ray of 69mZn has no interference with any adjoining photopeak. A critical comparison of these two methods is given.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Instrumental Neutron Activation Analysis (INAA) is one of five primary methods recognized by the Comité Consultatif pour la Quantité de Matière (CCQM), and is often used by the National Institue for Standards and Technology (NIST) and the International Atomic Energy Agency (IAEA) for multielemental analysis of select trace and major elements [1]. INAA is also a non-destructive method, unlike ICP-MS, which is another major method for finding Zn in small samples less than 500 mg [2]. Zinc is potentially a difficult element to measure using INAA, depending especially on the ratio of zinc to scandium which is present in the sample. In the 1980s it was reported that zinc was difficult to determine in geological samples if the scandium to zinc ratio was approximatley the same [3]. In 1990 an exhaustive comparison of 160 geological samples with ICP-MS and NAA, found lack of sensitivity of activation analysis for zinc and other elements [4].
Zinc has a low absorption cross section, especially compared to scandium and europium, both of which can interfere with the major 65Zn photopeak at 1115.5 keV. Natural Zn is made up of 64Zn, 66Zn, 67Zn, 68Zn, and a trace of 70Zn. Radiative capture by 66Zn and 67Zn will lead to stable isotopes, and there are no other predominant reactions with neutrons of thermal or epithermal energy that produce radioactive isotopes. The isotope 70Zn is less than 1 % of natural Zn and does not have a high radiative capture cross section. The predominant neutron absorption reactions of 64Zn and 68Zn are radiative capture. We identified both of these isotopes in order to determine which is the best to use to measure zinc concentrations in geological samples by NAA. [5].
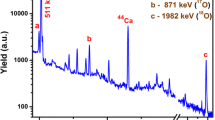
The isotope 64Zn, which makes up 49 % of natural zinc, captures a neutron to become 65Zn, which has a half- life of 244 days. It has three decay energies, but only the decay energy of 1115.5 keV has a high enough intensity to be detected at 50.6 % [5]. The difficulty in measuring this peak in geological samples comes from the presense of 45Sc in most geological samples, which is the only naturally occuring scandium isotope. When irradiated, 45Sc becomes 46Sc, which has an 84 day half -life. Scandium has a major peak at 1120.5 keV, which has a much higher intensity than the 1115.5 keV 65Zn decay peak at just under 100 % [5]. Scandium also has a much higher radiative capture cross section than 64Zn, which can be seen in Fig. 1. On the other side of 65Zn peak on the energy spectrum, a 152Eu peak at 1112.1 keV also causes some interference with the 65Zn decay peak. However, the 151Eu from which it is derived is 49 % of the natural abundance of europium, the intensity of the peak is only 37 %, and europium is usually present at lower levels than zinc or scandium. Also, the 152Eu has a 13.5 year half-life, so it decays at a much slower rate than 65Zn, so the interference is less significant than the 46Sc interference. However, 151Eu has a very high radiative capture cross section, as can be seen in Fig. 1. This triplet of 1112.1, 1115.5 and 1120.5 keV gammas can be very challenging to correctly fit in any peak fitting routines, and so the ability to accurately measure zinc from this peak is not only determined by the resolution of the detector, but the peak fitting algorithm as well.
Radiative capture cross section versus neutron energy of 64Zn (light grey, bottom line), 45Sc (dark grey, middle line), and 151Eu (dark grey, top line). [5]
Based on the properties of the 65Zn peak, we expect the detection of zinc to be optimized by using epithermal neutrons and by using a Compton suppression system. It can somewhat be seen from Fig. 1 that the radiative cross section for 64Zn is actually higher for higher energy neutrons than for thermal neutrons, while 45Sc and 151Eu have a much higher radiative cross sections for thermal rather than epithermal neutrons. In fact the ratio of the thermal cross section to the resonance integral for 64Zn is 1.73 ± 0.09, while for 45Sc it is 0.44 ± 0.02 and for 151Eu it is 0.26 ± 0.06 [6]. This means that the peak will be enhanced for 65Zn and suppressed for 46Sc and 152Eu when the samples are irradiated with epithermal neutrons.
The 46Sc gamma ray of 1120.5 keV and the 151Eu gamma ray at 1112.1 keV are both in coincidence with other gamma rays, while the 65Zn gamma ray at 1115.5 keV is not in any conicidence. A Compton suppression system, which is primarily used to reduce background counts in spectra due to Compton scattering, will suppress any coincident gamma rays [7, 8]. Therefore the use of a Compton suppression system and epithermal neutrons should give the best measurement of the 65Zn peak, because of the better ratios of the 65Zn peak to both the 46Sc peak and the 152Eu peak. However, ultimately the accuracy of the detemination of the 1115.5 keV gamma is a function of the resolution of the detector in conjunction with the peak-fitting algorithms.
The isotope 68Zn makes up 19 % of natural zinc, and captures a neutron to become 69Zn, which will decay with a half-life of 56 min, or will decay from a metastable state of 69mZn with a half-life of 13.8 h. The stable decay emits a gamma ray with almost no intensity, but the metastable isotope decays 99.97 % of the time by isomeric transition to stable 69Zn by a gamma ray of 438.6 keV with an intensity of 94.8 % [5]. Since this decay energy is within the Compton region of the spectra, the background counts will be high, especially for geological samples which have many different photpeaks with a range of energies all contributing to the continuum. Since this gamma ray is not in coincidence with other gamma rays, the Compton suppression system can be used to increase the peak to background ratio.
Experimental
In order to determine the best technique for measuring zinc using INAA, the concentration of zinc in NIST coal 1632c, soil NIST 2709, and fly ash NIST 1633a was measured by using three different techniques on each type of sample. Each sample was made in duplicate. Samples were weighed and put into polyethylene containers along with aluminum or molybdenum wires to compare the neutron flux of each sampel. Calibration was done using a liquid standard with a concentrations of 100 ± 0.48 μg/g of zinc. A gram or less of each type of NIST sample was also dried in a 105 °C oven over night and weighed before and after so that the masses could be corrected for moisture content. Irradiations were done at the University of Texas at Austin in a 1 MW TRIGA reactor. The irradiated samples were counted with an ORTEC Gamma-X germanium detector with an efficiency of 32.7 % and FWHM of 2.0 keV at 1.33 MeV 60Co source, and a Na(I) detector used for the Compton suppression system [8]. The different methods used are summarized in Table 1. The neutron fluxes given are estimated based on a flux of 4.5 × 1012 neutrons cm−2 s−1 at the reactor’s maximum power of 1 MW. To get an epithermal neutron flux, the samples are put in a tube lined with cadmium, which reduces the overall flux by about 90 %.
Results and discussion
The results can be seen in Table 2. The results show very good agreement with the certified values, with all measurements falling within the certified range and all deviations lower than 15 %. It is clear from the results that the concentration measurements based on the 65Zn peak had lower uncertainty and detection limits than the measurements based on the 69mZn peak. However, despite the higher detection limits, at these zinc concentratations this method using the 69mZn peak is still viable for the measurement of zinc.
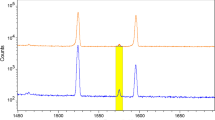
In Fig. 2, a spectrum from a short irradiation of NIST 1633a shows the 69mZn peak at 438.6 keV. When comparing this to Fig. 3, which is of both long irradiations of NIST 1633a showing the 65Zn peak at 1115 keV, it is clear that the peak of 69mZn has a much lower peak to background ratio than the 65Zn peak. In Fig. 3, the 65Zn peak is not quite as well separated from the 46Sc or 152Eu photopeaks for the thermal spectrum as it is for the epithermal spectrum. The insuffitient separation of the overpowering 46Sc peak may explain why the measurement of the 65Zn peak in 1633a ends up with extra counts and an overestimation of the concentration of zinc, as can be seen in Table 2.
If it is known that the sample has fairly low levels of zinc, scandium, and europium, then it is clear from Table 2 that either thermal or epithermal neutrons can be used to get very good measurements of the zinc concentration providing Compton suppression is employed. When comparing our results to the certified values, all results from the epithermal neutron irradiation gave results that had low uncertainties and deviations. The thermal neutron irradiation also gave very good results with even lower deviations for NIST 1632c and NIST 2709, except that the deviation for NIST 1633a is fairly high, with measurements of around 133 and 136 μg/g. This overestimation may be due to due to the 46Sc photopeak. NIST 1633a has 40 μg/g of scandium, which is far more than NIST 1632c or NIST 2709, with around 3 and 12 μg/g of scandium respectively. While the separation of the peaks for thermal are still fairly good for the 1633a, the counts between the three peaks do not go quite down to background, as can be seen in Fig. 3. Even though there is more than five times more zinc in the sample than scandium, the 46Sc peak still overpowers the 65Zn peak. The epithermal irradiation reduces this effect quite a bit. For the NIST 1633a peaks, the ratio of scandium to zinc is around 13 for thermal neutrons, and only 5.5 for epithermal. This reduction in peak area ratio should give better results for samples with significant scandium content. This may explain our results, but it is hard to say for certain without more sample repetition.
Conclusion
INAA is often used for multi-element analysis, and is capable of measuring a large number of different elements. Having a reliable INAA method for measuring zinc concentrations increases the usefulness of INAA as a technique. All three methods are in very good agreement with the certified NIST values. However,. if there are low levels of zinc and high levels of scandium or europium are present, using a long irradiation with epithermal neutrons and a Compton suppression system will likely give the most reliable measurement of zinc concentrations.Using the 69mZn isotope with epithermal neutorns yields good results but the detection limits are higher than using either thermal or epithermal neutrons and 65Zn. While detector resolution and good peak fitting programs are essential for determining zinc the ratio of the 46Sc 1120.5 photopeak to the 65Zn 1115.4 keV photopeak is also an important consideration.
References
Greenberg RR, Bode P, De Nadai Fernandes EA (2011) Spectrochim Acta Part B 66:193–241
Makishima A, Kobayashi K, Nakamura E (2002) Geostand Newsl 26:41–51
Graham CC, Glascock MD, Carnl JJ, Vogt JR, Spalding TG (1982) Anal Chem 54:1623–1627
Hall GE, Bonham-Carter GF, Maclaurin AI, Ballantyne SB (1990) Talanta 37:135–155
Table of Nuclides (2000) Nuclear Data Center, Korea Atomic Energy Research Institute. http://atom.kaeri.re.kr. Accessed 13 March 2012
Mughabghab SF (2006) Atlas of neutron resonances: resonance parameters and thermal cross sections Z = 1-100. Elsevier Science, The Netherlands
Landsberger S (1994) J Radioanal Nucl Chem Art 179:67–79
Ahmed YA, Landsberger S, O’Kelly DJ, Braisted J, Gabdo H, Ewa IOB, Umar IM, Funtua II (2010) App Radiat Isot 68:1909–1914
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canion, B., Landsberger, S. Determination of zinc in geological samples using Compton suppression with thermal and epithermal instrumental neutron activation analysis. J Radioanal Nucl Chem 296, 379–382 (2013). https://doi.org/10.1007/s10967-012-2125-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2125-z