Abstract
Tritium and other stable isotopes in precipitation were analyzed on a monthly based on Jeju Island and in Daejeon Korea and variations between the island and continent were compared. The tritium concentration in Daejeon ranged from 2.27 to 15.71 TU and on Jeju from <0.5 to 5.4 TU. The maximum value of the tritium content was in March and the minimum in July and August due to the dilution effect of heavy rain. The tritium content in precipitation on Jeju Island was lower than in Daejeon and the results reflected the general tritium content value in the Northern Hemisphere. The stable isotope analysis results showed that the mean value of δ 18O (‰) was −6.28 and ranged from −11.70 to −1.67. Further the mean δD (‰) value was −36.33 and ranged from −85.56 to 4.27. The mean deuterium excess value (d-value) was 13.89 ‰ and ranged from 3.33 to 33.61 ‰.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various isotopes are used in the fields of geosciences and environmental studies. Tritium is produced mainly by the interaction of cosmic radiations with the upper atmosphere, at a rate of about 0.25 atoms/cm2/s [1]. This tritium enters the hydrological cycle in the form of tritiated water molecules (HTO). Tritium content of water sample is expressed as tritium unit (TU), where one TU represents one THO molecule in 1018 H2O molecules and this means 0.11919 ± 0.00021 Bq/kg of water. Tritium activity measurement in environment is very difficult due to its very low concentration such as 10–20 TU at the Northern Hemisphere and below 10 TU at the Southern Hemisphere. To overcome this difficulty, the most of the water samples were enriched using electrolytic enrichment method.
The stable isotopes 18O and deuterium (2H) are also rare components present in water. The isotopic ratio of 18O/16O and 2H/1H were different in the environment due to their kinetic isotopic process, equilibrium fractionation process and the physicochemical effect. These isotopes offer a broad range of possibilities for studying processes within the water cycle and have thus become an important tool in isotope hydrology, and are routinely applied in studies of the origin and dynamics of surface and groundwaters. To this end, the Global Network of Isotopes in Precipitation (GNIP) collect a database that containing data on δ 2H, δ 18O and 3H activity across the world, as well as relevant meteorological data on a monthly basis, providing an extremely valuable tool for modelling climatic changes, as well as for hydrological and hydrogeological investigations [2].
Tritium in water vapor has its origin in the stratosphere, flowing into the troposphere and reaching the ground in the form of water vapor or precipitation. Thus, the tritium concentration in precipitation varies with the season and meteorological characteristics at the observation station [3]. The isotopic composition of local precipitation is primarily controlled by regional-scale processes, like the trajectories of water vapor transport over the continents and the average rainout history of the air masses giving rise to precipitation in a particular place [4]. Stable isotopes can therefore be used to investigate precipitation formation conditions, and to monitor changes in these conditions, as well as their impact on water resources, and to relate these conditions to observed and expected climatic changes. For this reason, much research has been published on the seasonal and regional variations of stable isotopes and tritium [5–10].
In this study, we determine the monthly variability of the isotopic composition in precipitation between coastal and continental regions, as well as regional variation on Jeju Island, Korea.
Experimental
Sampling
Considering differences in direction and height, nine sampling sites were selected on Jeju Island, as shown in Table 1 and Fig. 1. Precipitation was also collected at Daejeon, Korea to compare isotopic variations between coastal and continental regions. Precipitation was collected for a month using a 20 L plastic bottle fitted with a conical stainless steel funnel. The bottle was wrapped in Al foil to keep the sample out of direct light and paraffin oil was poured into the bottle to avoid evaporation. The precipitation sample was collected every month and stable isotopes and tritium were analyzed from Dec. 2009 to Nov. 2010.
Reagents and instruments
Precipitation was filtered with 0.45 μm nuclearpore filter and transferred to a polyethylene bottle. A 1 L sample was distilled to remove impurities and 1 wt% GR grade Na2O2(97 %, Sigma–Aldrich) was added as an electrolyte. After electrolytic enrichment process which was discussed our previous work[11], the distilled 10 mL water sample was mixed with a 10 mL cocktail (Ultima Gold LLT, Perkin Elmer Co, USA) which was a mixture of scintillator, solvent and surfactant. The tritium measurement was performed with a Quantulus 1220 (Wallac, Perkin Elmer) low level liquid scintillation counter. Tritium counting efficiency was estimated using the NIST standard water sample(SRM 4926E, tritiated water). With a 10 mL sample and 300 min of counting time, the detection limit was determined 2.17 Bq L−1, which corresponds to about 18 TU. Therefore, most of the water samples could not be analyzed directly without enrichment.
The tritium free background water was acquired from a 500 m deep groundwater well located in the Korea Atomic Energy Research Institute. The age of the background water sample was determined to be about 2500 year by the C-14 counting method. The counting vial was a 20 mL polyethylene vial with its inner side coated with Teflon. The oxygen isotope was pretreated using the CO2–H2O equilibrium method and deuterium was pretreated using the zinc reduction method. Pretreated stable isotopic samples were analyzed by mass spectrometry (VG Prism II). The measured value was represented as a standardized permillage (‰) with the Vienna standard mean ocean water (VSMOW). The analyzed 18O and D values were represented as δ-value (‰),
where Rsample and Rstandard denote the D/H or 18O/16O ratios of the sample and standard, respectively.
Results and discussion
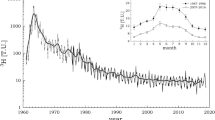
The monthly distribution of the tritium concentration at Jeju Island and Daejeon are shown in Fig. 2. The tritium concentration at Daejeon ranged from 2.27 to 15.71 TU and on Jeju from <0.5 to 5.4 TU. Figure 3 shows the spatial distribution of the tritium values. The maximum value of the tritium content was in March and the minimum value in the rainy season (Jul, Aug) due to the dilution effect of heavy rain. The H-3 content in the eastern and northern regions of Jeju Island was higher than those in the west and south. This result reflects the differences in the air mass and amount of rain across Jeju Island. Tritium contents in the precipitation on Jeju Island were lower than at Daejeon, and the results reflected the general tritium content value of the Northern Hemisphere [2].
Spatial and temporal variations in the isotopic composition of precipitation are due to isotopic fractionation that occurs during the evaporation of seawater and condensation during the advection of water vapor [12]. Previous research [9–14] has pointed out that high tritium concentrations are related to the arrival of continental air masses from the north, and that low tritium concentrations are related to the presence of oceanic air masses. Therefore, the H-3 content in precipitation on Jeju Island was representative of maritime characteristics.
The monthly distribution of stable isotopes is shown in Table 2 and Fig. 4. The stable isotope analysis results show that the mean δ 18O (‰) value was −6.28 and ranged from −11.70 to −1.67. The mean δD (‰) value was −36.33 and ranged from −85.56 to 4.27. The mean deuterium excess value (d-value), defined by Dangaard [12], was 13.89 ‰ and ranged from 3.33 to 33.61 ‰.
The regional variation in isotopic content is shown in Fig. 5. Compared to other regions, the eastern region had the highest isotope value. The mean δ 18O (‰) value in the eastern region was −5.75 and ranged from −11.51 to −3.24. The mean δ D (‰) value was −30.1 and ranged from −82.20 to −11.11. The effect of elevation was also monitored. The isotopic content was high at sampling sites with elevation below 200 m and decreased above 600 m. This elevation effect was evident for the δ 18O values, but not for δD and d-value. The d-value on Jeju Island showed highs in winter and lows in summer. This result illustrated that precipitation on Jeju Island was affected from the North Pacific air mass at summer season and the Siberian air mass at winter season. The North Pacific air mass was produced from slow evaporation in high temperature and humidity, therefore, the precipitation d-value showed below 10 ‰. On the other hand, the Siberian air mass, which is cold and dry, was produced from fast evaporation by passing through the relatively warm Yellow Sea, it showed above 15 ‰ d-value. This result was in good agreement with previous others work [15, 16].
Figure 6 illustrates the relationship δD = f(δ 18O) for all sampling stations. The isotopic values for precipitation in the rainy season (Jun, Jul, Aug) was similar to the global meteoric water line but the value in the dry season (Dec, Jan, Feb) deviated from this. In spring and fall, the values fell between the dry and rainy seasons. The spatial distribution of stable isotope depends on a number of weather parameters that characterize the origin, vapor supply, and removal history of the air moisture that generates the precipitation. The seasonal differences in isotope values also arise from the air mass effect. The North Pacific air mass, which is of high temperature and humidity, affects precipitation in the summer season and Siberian air mass, which is cold and dry, affects precipitation in the winter season.
The precipitation with respect to the global meteoric water line of Craig [17]
Conclusion
This paper attempts to provide a comprehensive survey of the isotopic composition of precipitation over Jeju Island for stable isotopes (2H, 18O) and 3H, and the results are compared with continental values. The continental station (Daejeon) showed distinct differences in the isotopic composition of precipitation when compared to the maritime stations. The slope of the local meteoric water lines (LMWL) for Jeju Island, in the rainy season are not considered to be different from that of the GMWL (8 ‰) at a 95 % confidence level, while the LMWLs in the dry season have slopes lower than 8 ‰. The spatial distribution of the average d-value is not homogeneous, reflecting differences in the origin and history of the water vapor masses that generate precipitation.
References
Solomon DK, Cook PG (2000) In: Cook PG, ALed Herezeg (eds) Environmental tracers in subsurface hydrology. Kluwer Academic Publishers, Boston, p 397
IAEA/WMO, 2012. Global Network of Isotopes in Precipitation. The GNIP database http://www.univie.ac.at/cartography/project/wiser
Taylor CB (1966) Tellus 18:105–131
Rozanski K, Sonntag G, Munnich KO (1982) Tellus 34:142–150
Vreca P, Bronic IK, Horvatincic N, Baresic J (2006) J Hydrol 330:457–469
Fourré E, Jean-Baptistea P, Dapoigny A, Baumier D, Petit J-R, Jouzel J (2006) Earth Planet Sci Lett 245:56–64
Matsuoka N, Hirai E, Tagomori H, Momoshima N, Takashima Y (1994) Sci Total Environ 145:197–205
Palomo M, Penalver A, Aguilar C, Borrull F (2007) Appl Radiat Isot 65:1048–1056
Argiriou AA, Lykoudis S (2006) J Hydrol 327:486–495
Moon SE, Kim YK, Park JK (1992) Atmos Environ 26A:2751–2758
Yoon YY, Lee KY, Ko KS (2010) J Radioanal Nucl Chem 286:591–595
Dansgaard W (1964) Tellus 16(4):437–468
Moghissi AA, Porter CR (1970) Radiol Health Data Rep 3:137–140
Kigoshi K, Yoneda K (1970) J Geophys Res 75:2981–2984
Lee KS, Lee I, Choi MS, Brag EJ (1997) J Geol Soc Korean 33:139–147
Lee KS, Grundstein AJ, Wenner DB, Choi MS, Woo NC, Lee DH (2003) Clim Res 23:137–248
Craig H (1961) Science 133:1702–1703
Acknowledgments
This research was supported by the Basic Research Project (12-3211) of the Korea Institute of Geoscience and Mineral Resources (KIGAM) funded by the Ministry of Knowledge Economy of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ha, K., Yoon, Y.Y., Lee, K.Y. et al. Seasonal variation in the isotopic contents of precipitation in Korea. J Radioanal Nucl Chem 296, 389–395 (2013). https://doi.org/10.1007/s10967-012-2077-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2077-3