Abstract
In terms of pre-safety assessment of a potential site for high-level radioactive wastes disposal in China, the geochemical behavior of key radionuclides which tend to be released from the repository must be thoroughly investigated. 99Tc is a long-lived fission product with appreciable productivity in nuclear fuel, and Tc (+7) has unlimited solubility in near-field geochemical environments. In this study, the effects of ionic strength and humic acid on 99TcO4 − sorption and diffusion in Beishan granite were investigated with through-diffusion and batch sorption experiments. Results indicated that the effective diffusion coefficients (D e) of 99TcO4 − in Beishan granite varied from 1.07 × 10−12 to 1.28 × 10−12 m2/s without change with ionic strength, while the distribution coefficients (K d) negatively correlated with ionic strength of the rock/water system. This study also indicates that there is no evident influence of humic acid concentration on the diffusion behavior of 99TcO4 − in Beishan granite, due to the limited interaction between humic acid and 99TcO4 −.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Safety disposal of high-level radioactive wastes (HLW) is a worldwide challenge. So far, deep geological disposal is the most acceptable way in isolating the HLW from biosphere. To make sure the wastes can be safely isolated in a long time scale which is needed for the HLW radioactivity decays to the natural level, the pre-safety assessment of the repository is an essential task. And the investigation on the sorption and diffusion behaviors of key nuclides in geological barriers of the repository is an important aspect concerned in the pre-safety assessment.
In China, Beishan granite area in Gansu province has been pre-selected as one of the most potential research site for HLW repository [1]. Started from 1985, many research works have been performed in regard to site investigation, geologic survey, groundwater chemistry, core sample drilling, as well as radionuclide transport experiments [2–5]. For example, Lu et al. [6] investigated the diffusion of 125I− as a surrogate for 129I− in Beishan granite obtained at 300-m depth by laboratory diffusion experiments, and their results showed that the effective diffusion coefficient ranged from 2.44 × 10−12 to 2.72 × 10−12 m2/s. After this work, Chen et al. [7] investigated the influence of temperature on 125I− diffusion in 600-m deep Beishan granite, and found that the relationship between the effective diffusion coefficient and temperature could be described by the modified Nernst equation at 27–50 °C. For the pre-safety assessment of the potential site for HLW repository, lots of basic research work and fundamental data are still needed in China.
In the pre-safety assessment, 99Tc is one of the most important fission products in the spent fuels from nuclear power plants, and 99TcO4 − which has high mobility in geological environments is the predominant species under non-reducing condition. When 99Tc was released from the repository, it would transport through the geological barrier with groundwater and finally enter the biosphere and contribute to the total radiation dose. Although some reducing minerals exhibit relatively high absorption ability to 99Tc by reducing Tc(VII) to Tc(IV), a considerable amount of unreduced Tc(VII) still remains in the repository environment [8]. Therefore, TcO4 − which has the highest mobility is the possible form that can reach biosphere. Along the transport path, TcO4 − will interact with the geological medium under various hydrogeochemical conditions. In the past, numerous research works were performed to investigate the influence of redox potential on 99Tc diffusion in natural materials [9–15], while, other impact from pH, temperature and ionic strength were rarely considered. Actually, previous studies indicated that ionic strength could influence the sorption behavior of some radionuclides such as Cs, Eu, Se on natural materials [16–19]. On the other hand, when natural colloids are present in the water/rock system, it can trap some radionuclides and result in different migration behaviors with respect to the colloids-free systems [20–23]. In this work, the effects of ionic strength and humic acid on the sorption and diffusion behavior of 99TcO4 − in Beishan granite were investigated by batch-sorption and through-diffusion experiments.
Experimental
Materials
Beishan granite core sample was drilled out in the depth of about 600 m from Beishan area, Gansu province, China. The core sample was cut into a section of granite slices, which were 64 mm in diameter and 5 mm in thickness. Some fragment from the same granite sample was crushed and sifted by 200 mesh sieves to get granite powder sample, which was used for composition characterization and sorption experiments. The mineralogical composition of the granite sample was determined by XRD method, and the data is given in Table 1. The chemical and elemental composition of the granite sample characterized by X-ray fluorescence spectrometer (XRFS) (ARL ADVANT XP+, Thermo Electron Co.) is given in Table 2. The porosity values of the granite slices were measured by water immersion technique [6, 7, 14]. Table 3 shows the physical parameters of the granite slices. 99Tc was in the form of NH4TcO4 (Eckert & Ziegler Isotope). Humic acid produced from coal was bought from Sinopharm Chemical Reagent Co. Before experiments, it was dissolved in 0.1 M NaOH and then deposited upon addition of 0.3/0.1 M HCl–HF solution for purification. The elemental composition of the humic acid was determined by elemental analyzer (vario EL, Elementar Analysensysteme GmbH). The result is shown in Table 4. All other chemicals were of analytical grade and bought from Sinopharm Chemical Reagent Co.
Through-diffusion experiments
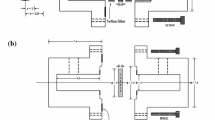
Through-diffusion technique was used to investigate the diffusion behavior of 99Tc in Beishan granite. The diffusion device was detailedly depicted in our pervious works [6, 7, 14]. Setup of the diffusion cell is shown in Fig. 1. It contained a larger source cell (~1,800 mL) and a smaller sampling cell (~70 mL). A granite slice was mounted between the two cells so that the granite slice was the only path connecting the two cells. The area of the effective cross section of the granite slice is 24.9 ± 0.4 cm2. During the experiments, the two cells were strictly sealed to avoid any possible water loss through evaporation.
Set-up of the diffusion cell [7]. 1. Sealing caps; 2. Sampling cell; 3. Granite slice; 4. Source cell; 5. Stirring bar
At the start of the experiments, 0.02/0.1/0.5 M NaClO4 solution which contained 0.0/1.0/10.0/20.0 mg/L humic acid was added into both cells as background solution The hydraulic heads in the source cell and the sampling cell were kept at the same level by monitoring the water levels with adding fresh background solution if necessary. Then a dose (~0.02 mCi) of 99TcO4 − solution was inserted to the source cell. After stirring for 1 day, 1.0 mL solution was sampled from each cell and then mixed with 10.0 mL scintillation cocktail (Optiphase Hisafe 2, Perkin Elmer), and the radioactivity of the mixture was measured with a liquid scintillation counter (Tri-Carb 3110TR, Perkin Elmer). The counting efficiency of 99Tc is 54.9 %. The specific activity of 99Tc in the source cell was measured as (5.0 ± 0.1) × 102 Bq/L. Meanwhile, 1.0 mL NaClO4 solution was injected into each cell. Thereafter, the activity of 99Tc in the sampling cell was measured in the same way at a 2-day interval for the first 10 days and at a 4-day interval for the rest of the 81-day.
Batch sorption experiments
Batch sorption experiments were carried out to investigate the sorption of 99TcO4 − on Beishan granite. The experiments were performed in polyethylene tubes under atmospheric condition at 25 °C. 0.30 g granite powder and 3.0 mL of 4.98 × 102 Bq/mL NH4TcO4 in 0.02/0.1/0.5 M NaClO4 solution were added into each tube. After shaking for 7 days, the equilibrium suspensions were centrifuged at 10,000 rpm for 30 min. The radioactivity of the supernatant was measured by the same method mentioned above.
Results and discussion
Diffusion behavior of 99TcO4 − in Beishan granite
As all of our experiments were performed at atmospheric condition, 99Tc should be in the form of 99TcO4 − without reduction [24, 25]. Since the concentration of 99TcO4 − in the sample cell was diluted after each sampling due to the replacement of 1.0 mL bulk solution by 1.0 mL background solution, the experiment data were corrected with the method used in a previous work [6, 7].
According to the experiment setup, the diffusion of radionuclide in the diffusion cell can be described by the decreasing inlet concentration-increasing outlet concentration through-diffusion model (DC–IC) or approximated by the constant inlet concentration-increasing outlet concentration through-diffusion model (CC–IC) [6, 7], and 99TcO4 − diffusion in granite can be described as:
where C(x,t) [cpm/mL] is the 99Tc concentration in pore water (represented as specific count rate) in the granite at time t [s] and at position x along the diffusion direction; D e [m2/s] is the effective diffusion coefficient for 99Tc; ε, ρ [kg/dm3] are the porosity and dry bulk density of the granite sample; λ is the decay constant of 99TcO4 −, which can be ignored for its negligible value of 1.04 × 10−13; S(x,t) [cpm/g] is the concentration (represented as specific count rate) of adsorbed 99TcO4 − on granite solid phase at time t and position x. S(x,t) can be described by linear adsorption model:
Therefore, Eq. (1) is converted into:
According to the experimental setup, Eq. (4) subjects to the following initial and boundary conditions:
where L [m] and A [m2] are the thickness and effective diffusion area of the granite sample, respectively; V u [m3] and V d [m3] are the volume of the source and sampling cell, respectively; C 0 [cpm/mL] is the initial concentration of 99TcO4 − in the source cell. C u(t) [cpm/mL] and C d(t) [cpm/mL] are the 99TcO4 − concentrations in the source and sampling cell at time t [s], which equal to C(0, t) and C(L, t) in value, respectively. Since V u ≫ V d, the decrease of C u is negligible.
In order to obtain the effective diffusion coefficient D e, experimental data is fitted with the diffusion Eq. (4) by a finite difference scheme. On the basis of the following assumptions:
Equation (4) can be converted into:
when Δt and Δx are chosen to satisfy Eqs. (9), (8) will have a stable numerical solution in the initial and boundary conditions indicated by Eqs. (5), (6), (7):
For each experiment, D e and K d were obtained by fitting the breakthrough curve of C d(t)/C 0 as a function of t when L, A, V u, V d and C 0 are given. Figure 2 shows the fitting result of one of the diffusion experiment. It indicates that the model well matches the present experimental data. The obtained D e and K d values were shown in Table 5. These values are comparable with the one obtained on other granites from the literatures (Table 6) [14, 15, 26]. The results indicated that there was no evident change on the D e values when ionic strength and humic acid concentrations were changed. The reason is that D e for TcO4 − depends on the diffusion behavior of TcO4 − in free water and the geometry of the micropore system in Beishan granite [27]. On the other hand, the K d values decreased when the ionic strength increased, implying that the interaction between TcO4 − and granite surface was weak electrostatic interaction. When the ionic strength is increased, more anions will involve in competitive sorption, which lead to reduction in TcO4 − sorption. Nevertheless, results in this study indicated that humic acid can not influence the sorption of TcO4 − on Beishan granite. Humic acid is a kind of organic compound contains lots of carboxyl and phenolic hydroxyl. Cations can easily be sorbed on humic acid, while anionic TcO4 − is hardly sorbed by humic acid [28]. In addition, the contribution of humic acid on ionic strength in our experiment was negligible compared to NaClO4. Therefore, the ionic strength could be considered as constant. Consequently, the influence of humic acid on TcO4 − sorption is negligible.
Sorption behavior of 99TcO4 − on Beishan granite
In the sorption experiments, the distribution coefficients K d′ [kg/L] are calculated by the following equation:
where V [L] is the volume of the solution containing 99TcO4 −; m [kg] is the mass of granite powder; c 0 [cpm] is the count of 1 mL 99TcO4 − solution before sorption and c [cpm] is the count of 1 mL supernatant after sorption. The result from the sorption experiments was shown in Table 5. The change trend of K d′ with ionic strength was similar to the result from the through-diffusion experiments. It confirmed that increase in ionic strength would reduce the sorption of TcO4 − on Beishan granite. But the K d′ values obtained from the sorption experiments were one order of magnitude higher than the one obtained from diffusion experiments. This can be attributed to the difference in solid size, since the granite used in the sorption experiments was crushed, while intact granite was used in the diffusion experiments [29]. From the results of this work, it can be deduced that when an anionic radionuclide species such as 99TcO4 − migrates to an area where the porewater has higher ionic strength, its mobility may be enhanced, even in the same host rocks. In order to predict the migration behavior of 99TcO4 − in the pre-safety assessment, the influence of ionic strength of the rock/water system must be taken into account. On the other hand, the influence of humic acid is negligible.
Conclusion
The effective diffusion coefficients (D e) of 99TcO4 − in Beishan granite obtained by through-diffusion experiments varied from 1.07 × 10−12 to 1.28 × 10−12 m2/s. Results indicated that ionic strength did not have evident effect on D e. The distribution coefficients (K d) of 99TcO4 − on Beishan granite obtained by through-diffusion experiments and batch sorption experiments negatively correlated with the ionic strength of the rock/water system. It was concluded that the interaction between TcO4 − and granite surface was weak electrostatic interaction. Due to the limited interaction between humic acid and 99TcO4 −, the effect of humic acid on the diffusion behavior of 99TcO4 − in Beishan granite is insignificant.
References
Wang J, Fan XH, Xu GQ, Zheng HL, Wang CZ, Fan ZW (2004) Geological disposal of high level radioactive waste in China: progress in the last decade (1991–2000). Atomic Energy Press, Beijing, pp 1–12
Xu GQ, Wang J, Jin YX, Chen WM (1995) Progress in site selection for China’s high level radioactive waste repository. In: Proceedings of the fifth international conference on radioactive waste management and environmental remediation, ICEM ‘95, p 755
Min MZ, Luo XZ, Wang J, Jin YX, Wang RC (2005) Sorption behavior of U(VI), U-234(VI) and U-238(VI) onto fracture-filling clays in Beishan granite, Gansu: application to selecting the site of high-level radwaste repository in China. Sci China Ser D Earth Sci 48(10):1649–1655
Sun ZX, Zhang ZS (2008) Geochemical modeling of water-granite interaction in Beishan area, NW-China. Geochim Cosmochim Acta 72(12):A917
Dong YH, Li GM, Li M (2009) Numerical modeling of the regional ground water flow in Beishan area, Gansu Province. Chin Sci Bull 54(17):3112–3115
Lu CJ, Liu CL, Chen T, Wang J, Wang XY, Su R, Sun JY, Yang RX, Zhang XS (2008) Determination of the effective diffusion coefficient for 125I(-) in Beishan granite. Radiochim Acta 96(2):111–117
Chen T, Sun M, Li C, Tian W, Liu X, Wang L, Wang X, Liu C (2010) The influence of temperature on the diffusion of (125)I(-) in Beishan Granite. Radiochim Acta 98(5):301–305
Vinsova H, Vecernik P, Jedinakova-Krizova V (2006) Sorption characteristics of Tc-99 onto bentonite material with different additives under anaerobic conditions. Radiochim Acta 94(8):435–440
Um W, Serne RJ (2005) Sorption and transport behavior of radionuclides in the proposed low-level radioactive waste disposal facility at the Hanford site, Washington. Radiochim Acta 93(1):57–63
Wang XK, Tan XL, Ning QL, Chen CG (2005) Simulation of radionuclides Tc-99 and Am-243 migration in compacted bentonite. Appl Radiat Isot 62(5):759–764
Winkler A, Bruhl H, Trapp C, Bock WD (1988) Mobility of technetium in various rocks and defined combinations of natural minerals. Radiochim Acta 44–5:183–186
Oscarson DW, Hume HB, Choi JW (1994) Diffusive transport in compacted mixtures of clay and crushed granite. Radiochim Acta 65(3):189–194
Szanto Z, Svingor E, Molnar M, Palcsu L, Futo I, Szucs Z (2002) Diffusion of H-3, Tc-99, I-125, Cl-36 and Sr-85 in granite, concrete and bentonite. J Radioanal Nucl Chem 252(1):133–138
Liu CL, Wang XY, Li SS, Wang ZM, Gao HC, Li B, Wen RY, Wang HF, Tang LT, Xin CT, Jiang L (2001) Diffusion of Tc-99 in granite: a small scale laboratory simulation experiment. Radiochim Acta 89(10):639–642
Liu DJ, Fan XH, Yao J, Wang B (2006) Diffusion of Tc-99 in granite under aerobic and anoxic conditions. J Radioanal Nucl Chem 268(3):481–484
Tsai SC, Wang TH, Li MH, Wei YY, Teng SP (2009) Cesium adsorption and distribution onto crushed granite under different physicochemical conditions. J Hazard Mater 161(2–3):854–861
Guo ZJ, Chen ZY, Wu WS, Liu CL, Chen T, Tian WY, Li C (2011) The adsorption of Eu(III) on Beishan granite. Sci China Ser Chem 41(5):907–913
Guo ZJ, Chen ZY, Wu WS, Liu CL, Chen T, Tian WY, Li C (2011) Adsorption of Se(IV) onto Beishan granite. Acta Phys Chim Sin 27(9):2222–2226
Papelis C (2001) Cation and anion sorption on granite from the Project Shoal Test Area, near Fallon, Nevada, USA. Adv Environ Res 5(2):151–166
Sen TK, Khilar KC (2006) Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv Colloid Interface Sci 119(2–3):71–96
Schmeide K, Bernhard G (2009) Redox stability of neptunium(V) and neptunium(IV) in the presence of humic substances of varying functionality. Radiochim Acta 97(11):603–611
Satmark B, Albinsson Y (1992) Sorption of fission-products on colloids made of naturally-occurring minerals and the stability of these colloids. Radiochim Acta 58–9:155–161
Degueldre C, Ulrich HJ, Silby H (1994) Sorption of am-241 onto montmorillonite, illite and hematite colloids. Radiochim Acta 65(3):173–179
Lieser KH, Bauscher C (1987) Technetium in the hydrosphere and in the geosphere. 1. Chemistry of technetium and iron in natural-waters and influence of the redox potential on the sorption of technetium. Radiochim Acta 42(4):205–213
Alliot I, Alliot C, Vitorge P, Fattahi M (2009) Speciation of technetium(IV) in bicarbonate media. Environ Sci Technol 43(24):9174–9182
Bradbury MH, Green A (1985) Measurement of important parameters determining aqueous phase diffusion rates through crystalline rock matrices. J Hydrol 82(1–2):39–55
Lofgren M, Neretnieks I (2003) Formation factor logging by electrical methods—Comparison of formation factor logs obtained in situ and in the laboratory. J Contam Hydrol 61(1–4):107–115
Kumar S, Rawat N, Kar AS, Tomar BS, Manchanda VK (2011) Effect of humic acid on sorption of technetium by alumina. J Hazard Mater 192(3):1040–1045
Andre M, Malmstrom ME, Neretnieks I (2009) Determination of sorption properties of intact rock samples: new methods based on electromigration. J Contam Hydrol 103(3–4):71–81
Acknowledgments
The project was jointly supported by the National Natural Science Foundation of China (Grant No. 10775008, 11075006, 91026010), Special Foundation for High-level Waste Disposal (2007-840), the Fundamental Research Funds for the Central Universities, Analysis foundation of Peking University (13–18) and the 111 projects, we acknowledge them for their financial supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, C., Wang, C.L., Liu, X.Y. et al. Effects of ionic strength and humic acid on 99TcO4 − sorption and diffusion in Beishan granite. J Radioanal Nucl Chem 293, 751–756 (2012). https://doi.org/10.1007/s10967-012-1746-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1746-6