Abstract
99Tc is an important radionuclides related to repository safety assessment. The mobility pertechnetate (TcO4 −) can be reduced to immobility technetium(IV) hydrous oxides (TcO2·nH2O) by Fe(II)-bearing minerals. In China, Gaomiaozi (GMZ) bentonite is regarded as the favorable candidate backfilling material for the HLW repository, which is contained some FeO. The diffusion behavior of 99Tc was investigated in GMZ bentonite by through- and out-diffusion methods. The effective diffusion coefficient (D e), the accessible porosity (εacc), apparent diffusion coefficient (D a) and distribution coefficient (K d) were decreased with the increasing of dry density. The D e values were (2.8 ± 0.2) × 10−11 m2/s and (3.5 ± 0.2) × 10−12 m2/s at dry density of 1,600 and 1,800 kg/m3, respectively. It was indicated that the dominating species was TcO4 − during the diffusion processing. While, out-diffusion results showed that part of TcO4 − may be reduced by Fe(II). The relationship of D e and εacc could be described by Archie’s law with exponent n = 2.4 for 99Tc diffusion in GMZ bentonite. Furthermore, the relationship between D a and dry density (ρ) was exponential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
China has the biggest reservation of bentonite in the world. Considering the mining scale, quality, economic limitation and location, Gaomiaozi (GMZ) bentonite was chosen as the potential backfilling materials for the HLW repository. It is in the northern Chinese Inner Mongolia autonomous region. There are 160 million tons with 120 million tones Na-bentonite reserves in the deposit and the mine area is about 72 km2. The deposit is formed in the late Jurassic [1, 2]. Compared to relatively well studies reference bentonite, such as such as MX-80 (USA, Switzerland and Sweden), Fo-Ca (France), FEBEX (Spain) and Kunigel V1 (Japan), the mineralogy composition of GMZ bentonite is different, whereas the chemical composition is similar to Kunigel-V1 [3]. The main composition is montmorillonite, which is built from layers of oxygen atoms and cations in tetrahedral–octahedral–tetrahedral (TOT) coordination. The structure has an excess of negative charge due to isomorphic substitution of Al for Si in tetrahedral layers and Mg for Al, or Li for Mg in octahedral layer. The negative surface is compensated by cations between the clay layers in the interlayer space [4]. It has been widely accepted that diffusion of anions in compacted bentonite is predominantly governed by pore water diffusion and anion exclusion effect. Anion exclusion was ineffective when the compacted dry density above 1,800 kg/m3 [5].
99Tc is a radioactive fission product of 235U and 239Pu and an important radionuclides related to repository safety assessment due to its long half-life (2.1 × 105 year), high environmental mobility and it possible uptake into the food chain [6]. 99Tc speciation, solubility and sorption behavior is strongly dependent on its valence state [7]. Under oxic and suboxic environments, the dominant form of Tc(VII) is pertechnetate (TcO4 −), which is highly solubility in water and essentially nonadsorptive properties toward sediment minerals. However, under anoxic conditions, Tc(VII) can be reduced to technetium(IV) hydrous oxides, i.e., TcO2·nH2O, which is sparingly soluble in water under circumneutral pH conditions [8]. Tc(VII) can be reduced to Tc(IV) by ferrous iron as either aqueous (Fe2+) or solid [Fe(II)], which is associated with solid minerals such as magnetite, hematite, goethite, etc. [9–11].
Diffusion coefficient is an important parameter for transport modeling in evaluating the safety of repository. The apparent diffusion coefficient in bentonite of Tc(VII) is in the range of 10−10–10−11 m2/s, whereas Tc(IV) is 10−12–10−14 m2/s [12–14]. The Fe(II)-bearing minerals like magnetite in granite could markedly slow the mass transport of 99Tc by reduction of Tc(VII) to Tc(IV), the apparent diffusion coefficient (D a) was decreased from 2.0 × 10−11–1.9 × 10−13 m2/s [15]. Whether this applies also to GMZ bentonite, which contains 0.29 % FeO [3], the diffusion behavior of 99Tc(VII) was investigate for the first time by through- and out-diffusion methods.
Materials and methods
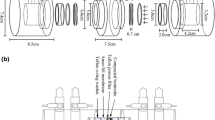
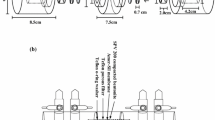
Through- and out-diffusion experiments were carried out under atmospheric condition using GMZ bentonite (contains 75.4 wt% montmorillonite) from Beijing research institute of uranium geology without further processing. GMZ bentonite powder was pressed into a cylinder about 2.54 ± 0.01 cm diameter and 0.87 ± 0.01 cm thick. The dry densities were 1,600 and 1,800 kg/m3, respectively. All experiments were carried out in artificial pore water of Gansu Beishan at room temperature. 0.003 M NaN3 was added in the pore water to stop bacterial growth.
The diffusion set-up and experimental procedure were the same as that of used for through- and out-diffusion experiments [16]. For through-diffusion experiment, NH4 99TcO4 was put in a source reservoir with 200 mL, whereas the target reservoir had a volume 20 mL. Both out-reservoirs had a volume 20 mL for out-diffusion. The samples were measured by liquid scintillation counting (Perkin Elmer Tri-Carb 3170 TR/SL). 5 mL of sample was placed in a 20 mL polyethylene counting vial and 13 mL scintillation cocktail (Packard LLT USA) was added. The counting efficiency was 100 ± 0.05.
The experimental and theoretical data processing in through- and out-diffusion methods has been described previously [17]. A house-made computer code compiled by mathematica 6.0 named fitting for diffusion parameters (FDP) was used for the experimental and modeling data processing [16, 18].
Results and discussion
Figure 1 showed the accumulated activity (A cum) as a function of diffusion time obtained by through-diffusion experiments at 1,600 and 1,800 kg/m3 dry densities, respectively. A cum were increased with time relatively slow at the transient states, becoming a linear function of time at the steady phase. A cum were increased faster at 1,600 kg/m3 than that at 1,800 kg/m3. D e and rock capacity factor (α) were obtained by fitting the experimental data of A cum versus t including both transient and steady-state phases.
Table 1 summarized the parameters used to fit the diffusion profile and the diffusion parameters results. V 0,dead and V l,dead are the dead volumes at the source- and out-reservoir sides, respectively. They contained the volume of the tubings, the grooves in the end plates and the filter pore space. D w (m2/s) is the bulk water diffusion coefficient. D w values was 1.95 × 10−9 m2/s [19]. K d, D a and geometrical factor (G) were calculated by following equations:
where εtot (−) is the total porosity obtained from the diffusion results of HTO in GMZ bentonite [16]; ρ (kg/m3) is the bulk dry density; δ is the constrictivity factor, which represents the reduction in diffusivity due to the pore narrowing; τ is the tortuosity factor, which represents the reduction of diffusivity due to the path lengthening [20]. Because of the anion exclusion, K d values were less than zero. It was higher at 1,600 kg/m3 than that of at 1,800 kg/m3. εacc, D e and D a decreased with the increasing of dry density. The D e values were 10−11–10−12 m2/s, indicating that the dominating species was soluble TcO4 −. D e and G values were about 10 and 7 times higher at 1,600 kg/m3 than that at 1,800 kg/m3. Similar results can be found elsewhere [21]. It indicated that the pore was narrowed or the path of TcO4 − was lengthened when GMZ bentonite was pressed, because εacc decreased only three times, δ might decreased or τ might increased when the dry density increased from 1,600 to 1,800 kg/m3.
Figure 2 showed flux as a function of diffusion time. The flux increased with diffusion time at the transient phase and became constant at the steady phase. The experimental data of flux as a function of diffusion time were in good agreement with the modeled data. It showed that flux of 99Tc was higher at 1,600 kg/m3 than at 1,800 kg/m3. It took circa 3 days to reach the steady state at 1,600 kg/m3, while it took longer time (circa 7 days) to reach it at 1,800 kg/m3.
Figure 3 showed the flux as a function of diffusion time obtained by the out-diffusion experiments. The shaded area represented the uncertainty of the calculated curve. The first experimental data did not fit well with the modeled data at out-reservoir sides because of the activity in dead volume, the uncertainty of the solution volume and the measurement. At low-concentration side, the experimental and theoretical data were agreed with each other well, whereas the experimental data were systematically higher than theoretical data at high-concentration side. The slow release of 99Tc resulted in the higher diffusive flux. Since the high dry density of bentonite was employed in the experiments, the heterogeneous porosity at the clay boundaries can be neglect [22]. It can be explained that that the species 99Tc may be changed during the diffusion processing. Because the GMZ bentonite used in the experiments were untreated, it may contain Fe-bearing minerals. Some 99TcO4 − could be transformed to insoluble TcO2·nH2O. Similar results can be found the in situ diffusion experiments of 99TcO4 − in borehole laboratory [23]. However, the reactions happed in this procedure is unclear, further investigation is underway to clarify this phenomenon.
Figure 4 showed the D e as a function of εacc. Here, εacc equals α for anion. For a given type of porous medium, D e can be related to D w and εacc by an empirical relationship analogous to Archie’s law [21, 24]:
where n is the cementation factor and has a constant value related to a given type of porous medium. Our literatures search showed that a few studies of 99Tc diffusion in bentonite were focused on both D e and εacc. The diffusion behavior of 99Tc were compared between GMZ and Czech R-bentonite [25] in order to study the relationship of D e and εacc. It could be described by Archie’s law with exponent n = 1.2–2.8 in bentonite, whereas n was equal to 2.4 in GMZ bentonite.
Previously research showed that an exponential relationship could be described between D a and dry density for 99Tc [14]. Figure 5 showed the relationship of the D a as a function of dry density in GMZ and other types of bentonite. D a and dry density could be described as the exponential relationship with intercept = 5.61 × 1010 and slope = −2.91 × 10−13. The linearly dependent coefficient R 2 was equal to 0.629. Then, it was showed that 99Tc had similar diffusion behavior in GMZ and other types of bentonite like MX-80, Avonlea etc. [13, 25–28], except that the species could be changed by Fe(II)-bearing minerals in the GMZ bentonite. However, little investigation was mentioned in other bentonite. Therefore, the diffusion parameters (D e and D a) of 99Tc in other bentonite could be proposed in the safety assessment for Chinese repository when there is lack of experimental results of GMZ bentonite.
Conclusions
The diffusion behavior of 99Tc in GMZ bentonite was investigated by through- and out-diffusion methods at dry density of 1,600 and 1,800 kg/m3. D e and α were obtained by modeling both through- and out-diffusion experimental data with a computer code FDP. D e, D a and εacc were decreased with the increasing of dry density. Out-diffusion results showed that the experimental data were higher than that of theoretical data at the source-reservoir side. It could be explained that the species of 99Tc may be changed when they were diffused in Fe(II)-bearing minerals in the GMZ bentonite.
The relationship of D e and εacc can be explained by Archie’s law with exponent n = 2.4 for 99Tc diffusion in GMZ bentonite. The effective diffusion coefficient of anion can be estimated from the knowledge of both εacc and the corresponding exponent n. Furthermore, the relation between D a and ρ was exponential. 99Tc had similar diffusion behavior in GMZ and other types of bentonite like MX-80, Avonlea, Kunigel V1, etc.
References
Wen ZJ (2008) Selection and basic properties of the buffer material for high-level radioactive waste repository in China. Acta Geo Sin Eng 82:1050–1055
Ye WM, Cui YJ, Qian LX, Chen B (2009) An experimental study of the water transfer through confined compacted GMZ bentonite. Eng Geol 108:169–176
Ye WM, Chen YG, Chen B, Wang QO, Wang J (2010) Advances on the knowledge of the buffer/backfill properties of heavily-compacted GMZ bentonite. Eng Geol 116:12–20
Sutton R, Sposito G (2001) Molecular simulation of interlayer structure and dynamics in 12.4 angstrom Cs-smectite hydrates. J Colloid Interface Sci 237:174–184
Kozaki T, Inada K, Sato S, Ohashi H (2001) Diffusion mechanism of chloride ions in sodium montmorillonite. J Contam Hydrol 47:159–170
Gu BH, Dong WM, Liang LY, Wall NA (2011) Dissolution of technetium(IV) oxide by natural and synthetic organic ligands under both reducing and oxidizing conditions. Environ Sci Technol 45:4771–4777
Icenhower JP, Qafoku NP, Zachara JM, Martin WJ (2010) The biogeochemistry of technetium: a review of the behavior of an artificial element in the natural environment. Am J Sci 310:721–752
Boggs MA, Minton T, Dong WM, Lomasney S, Islam MR, Gu BH, Wall NA (2011) Interactions of Tc(IV) with humic substances. Environ Sci Technol 45:2718–2724
Peretyazhko T, Zachara JM, Heald SM, Jeon BH, Kukkadapu RK, Liu C, Moore D, Resch CT (2008) Heterogeneous reduction of Tc(VII) by Fe(II) at the solid-water interface. Geochim Cosmochim Acta 72:1521–1539
Bishop ME, Dong HL, Kukkadapu RK, Liu CX, Edelmann RE (2011) Bioreduction of Fe-bearing clay minerals and their reactivity toward pertechnetate (Tc-99). Geochim Cosmochim Acta 75:5229–5246
Fredrickson JK, Zachara JM, Plymale AE, Heald SM, McKinley JP, Kennedy DW, Liu CX, Nachimuthu P (2009) Oxidative dissolution potential of biogenic and ablogenic TcO(2) in subsurface sediments. Geochim Cosmochim Acta 73:2299–2313
Sawatsky NG, Oscarson DW (1991) Diffusion of technetium in dense bentonite under oxidizing and reducing conditions. Soil Sci Soc Am J 55:1261–1267
Albinsson Y, Christiansensatmark B, Engkvist I, Johansson W (1991) Transport of actinides and Tc through a bentonite backfill containing small quantities of iron or copper. Radiochim Acta 52–53:283–286
Yu JW, Neretnieks I (1997) Diffusion and sorption properties of radionuclides in compacted bentonite. SKB Techinical Report 97-12
Oscarson DW, Hume HB, Choi JW (1994) Diffusive transport in compacted mixtures of clay and crushed granite. Radiochim Acta 65:189–194
Wu T, Dai W, Xiao GP, Shu FJ, Yao J, Li JY (2012) Influence of dry density on HTO diffusion in GMZ bentonite. J Radioanal Nucl Chem. doi:10.1007/s10967-011-1523-y
Van Loon LR, Soler JM, Bradbury MH (2003) Diffusion of HTO, 36Cl− and 125I− in opalinus clay samples from Mont Terri—effect of confining pressure. J Contam Hydrol 61:73–83
Wu T, Amayri S, Drebert J, Van Loon LR, Reich T (2009) Neptunium(V) sorption and diffusion in opalinus clay. Environ Sci Technol 43:6567–6571
Sato H, Yui M, Yoshikawa H (1996) Ionic diffusion coefficient of Cs+, Pb2 +, Sm3 +, Ni +2 , SeO4 2− and TcO4 − in free water determined from conductivity measurements. J Nucl Sci Technol 33:950–955
Loon LRV, Soler JM (2004) Diffusion of HTO, 36Cl−, 125I− and 22Na+ in opalinus clay: effect of confining pressure, sample orientation, sample depth and temperature PSI Bericht Nr04-03
Van Loon LR, Glaus MA, Muller W (2007) Anion exclusion effects in compacted bentonites: towards a better understanding of anion diffusion. Appl Geochem 22:2536–2552
Glaus MA, Frick S, Rossé R, Loon LRV (2011) Consistent interpretation of the results of through-, out-diffusion and tracer profile analysis for trace anion diffusion in compacted montmorillonite. J Contam Hydrol 123:1–10
Jansson M, Eriksen TE (2004) In situ anion diffusion experiments using radiotracers. J Contam Hydrol 68:183–192
Descostes M, Blin V, Bazer-Bachi F, Meier P, Grenut B, Radwan J, Schlegel ML, Buschaert S, Coelho D, Tevissen E (2008) Diffusion of anionic species in Callovo–Oxfordian argillites and Oxfordian limestones (Meuse/Haute–Marne, France). Appl Geochem 23:655–677
Vecernik P, Jedinakova-Krizova V (2006) Diffusion of 99-technetium in compacted bentonite under aerobic and anaerobic conditions. Czech Phys 56:D665–D672
Sato H, Ashida T, Kohara Y, Yui M, Sasaki N (1992) Effect of dry density on diffusion of some radionuclides in compacted sodium bentonite. J Nucl Sci Technol 29:873–882
Sawatsky NG, Oscarson DW (1991) Diffusion of technetium in dense bentonite. Water Air Soil Poll 57–58:449–456
Wang XK, Tao ZY (2004) Diffusion of 99 TcO4 − in compacted bentonite: effect of pH, concentration, density and contact time. J Radioanal Nucl Chem 260:305–309
Acknowledgments
The authors would like to thank for the productive discussions with Dr. Luc. R. Van loon, Paul Scherrer Institute, Switzerland. This work was financially supported by Qianjiang talents project in Zhejiang province and Project supported by the Scientific Research Starting Foundation for Returned Overseas Chinese Scholars, Ministry of Education, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J.Y., Dai, W., Xiao, G.P. et al. Pertechnetate diffusion in GMZ bentonite. J Radioanal Nucl Chem 293, 763–767 (2012). https://doi.org/10.1007/s10967-012-1733-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-1733-y