Abstract
Zircon is an accessory mineral, which occurs at low concentrations in a wide variety of rocks and is a host for hafnium, rare-earth elements (REE) and radio active elements like uranium and thorium. The presence of uranium in zircon has led to its increased use in the age determination of rocks. Zirconium is also considered as a strategic, hi-tech element because of its various applications, especially in the manufacturing, nuclear and aerospace industries. Analysis of zircon constitutes one of the tough tasks in analytical chemistry as it is a highly resistant mineral and it is extremely difficult to achieve its complete decomposition. In the present work, inductively coupled plasma mass spectrometry has been applied to the determination of hafnium, REE, uranium and thorium in zircon samples using two different sample dissolution procedures, one employing sodium peroxide fusion and another using a fusion mixture of KHF2 and NaF in 3:1 ratio. Some selected zircon samples originating from different places on the eastern coast of India have been analysed by both the methods and values obtained by both methods were found to be in good agreement with each other. Though a number of international zircon reference materials are available, certified or even proposed values are available only for a very few elements in them. Two zircon reference materials have also been analysed by both methods and usable values have been proposed in this paper. The values obtained by both methods were found to compare well with each other and as well with those reported in literature. The % RSD for all the estimated elements varied from 1.0 to 12.0% at different concentration levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zircon is a ubiquitous accessory mineral (ZrSiO4) found in a wide range of igneous, metamorphic, and clastic sedimentary rocks and is one among the most stable minerals found in nature. It has the ability to concentrate uranium and exclude lead from its crystal structure and this forms the basis of U–Pb geochronology [1]. Further, its refractory nature and concentric growth patterns ensure robust lasting records of crystallization age.
Zircon incorporates other important trace elements also besides U and Th into its structure, like the rare-earth elements (REE), Y, P and Hf. Although there is a complete solid solution between zircon and hafnium (HfSiO4), the Hf content of most natural zircons ranges from 1 to 3 wt% HfO2. The predominant trace elements incorporated into zircon, because of their similarity in ionic radius to Zr4+, are the heavy REE and Y. Natural zircon contains from ~1 to 20,000 ppm of uranium and from ~0.5 to 5,000 ppm of thorium. Use of the trace element distribution data in zircons for geological purposes is being reported [2]. Based on the incorporation of actinides, lanthanides, and other trace elements in zircon, Pupin developed geodynamic models for the emplacement of felsic rock suites [3]. REE analysis of zircon coupled with high spatial resolution U–Pb geochronology and imaging is emerging as a useful petrogenetic tool capable of providing a link between dated zircon growth and contemporaneous magmatic conditions or metamorphic reactions.
Further, zirconium has also emerged as a strategic, hi-tech element because of its various applications, especially in the manufacturing, nuclear and aerospace industries. In nuclear reactors, zirconium is primarily used in the cladding of the fuel rods and also in chemical piping in corrosive environments, heat exchangers, and in various special alloys because of its low neutron-capture cross-section and good corrosion resistance. Zircon sands find use in ceramics, refractories and foundries. All the above sited applications of zirconium have created a large demand for mining, separation and supply of high grade zircon (certified with chemical analysis data) from the heavy mineral deposits which occur in the beach sands of coastal areas.
In view of the above applications in geo-chemistry and industry it has become necessary to develop new and improved analytical methodologies for accurate and precise determination of various trace elements in zircon. But a survey of literature shows only a few publications on trace element determination in zircon samples. Various instrumental analytical techniques have been utilized for trace element analysis of zircons. Hironao Shinjoe et al. [4] have carried out trace element analysis of zircons from Japan by laser ablation-ICP-MS (inductively coupled plasma mass spectrometry). Hoskin [5] has determined minor and trace elements in natural zircon by SIMS and laser ablation ICP-MS and compared the results obtained by both techniques. Cesbron et al. [6] and Hanchar et al. [7] have used cathode-luminescence to study rare-earth distribution in zircons. Zinner and Crozaz [8] have determined REE using ion probe technique.
Solution ICP-MS is another powerful and sensitive technique that has been successfully used for the accurate direct determination of trace elements in geological materials. Literature on the analysis of zircon by solution ICP-MS is scarce. Perkins et al. [9] have reported analysis of zircon by laser ablation and solution ICP-MS.
Analysis of zircon by solution techniques poses a challenge to the analyst since it is a highly resistant mineral and it is extremely difficult to achieve its complete decomposition/dissolution using combinations of various mineral acids [10]. The mineral is only slightly attacked with hydrofluoric acid under atmospheric conditions on a steam water bath and requires vigorous conditions like elevated temperatures as high as 240 °C in closed Teflon lined steel bombs to bring about complete decomposition. The use of hydrofluoric and nitric acids for the digestion of silicate samples is well established, and has been applied to the dissolution of basalts, soils and other rocks. Use of this approach for the analysis of zircon is problematic since it is refractory and does not go into solution completely and the recovery of Zr and Hf is frequently only about 50%. Wu et al. [11] used HF, HNO3, H2O2 and H3BO3 to decompose NIST SRM 2704, NIST SRM 2709, NIST SRM 1646 and NIST SRM 2711 and obtained recoveries of Zr that were within about 50% of the certified value. Yoshida et al. [12] used HF, HNO3 and HClO4 to decompose six rock reference samples issued by the Geological Survey of Japan and observed that the analytical results for Zr in JG-1 were under estimated.
Some workers recommend the use of closed PTFE lined bombs and microwave dissolution techniques to achieve complete dissolution of zircon samples. Sample decomposition is complete because of the rigorous conditions employed with the added advantage of there being no loss due to volatilization since closed vessels are used in the bomb techniques. But the disadvantage with bomb techniques is that they are time consuming and cannot be easily applied when several hundred samples have to be analysed. Further, contamination of sample solutions can occur from the metallic parts of bombs. Very long cleaning and maintenance times are also involved. The analytical papers published thus far, which are based on the use of microwave to dissolve samples containing refractory minerals, have reported low recoveries for Zr and Hf [13]. Hence, fusion with fluxes is the preferred mode of sample dissolution for zircon analysis. Fusion procedures are fast, do not require expensive equipment and can effectively dissolve refractory mineral matrices as well. Various fusion fluxes have been utilized for the dissolution of zircon. Zircon is only partially decomposed with sodium carbonate flux. Mixture of Li2B4O7–LiBO2 has been recommended by several workers for the dissolution of zircon [14]. Both fusion and acid digestion methods have been employed by Balram and co-workers [15] for the analysis of zircons from southern coastal India by ICP-MS.
In the present work, ICP-MS has been applied to the determination of REE, uranium, thorium and hafnium in zircon samples originating from different places on the eastern coast of India, using two different sample decomposition procedures, one employing sodium peroxide fusion and another using a fusion with a mixture of KHF2 and NaF in 3:1 ratio. Though a number of international zircon reference samples are available, certified or even proposed values are available only for a very few elements in them. Two zircon reference materials have also been analysed and usable values have been proposed in this paper.
Experimental
Instrumentation
ICP-MS model: “Platform XS” from M/S G.V. Instruments/Micromass Ltd. (UK) which is fully computer controlled bench-top quadrupole ICP-MS was used for the determination of various elements. Instrumental operating conditions are given in Table 1. The instrument comprises of:
-
(1)
Sample introduction system for aqueous samples which includes Gilson Miniplus 3 peristaltic pump, a concentric glass low dead volume nebuliser, a quartz glass ICP torch and a water cooled spray chamber mounted on a cassette.
-
(2)
The ICP generator which is a solid state crystal controlled 27 MHz system, with RF power variable from 1,000 to 1,400 W. The ICP torch positioning is also computer controlled in X, Y and Z directions. Mass flow controllers are provided on all gas flows to the ICP.
-
(3)
Water cooled ICP ion-sampling interface comprising of an outer cone (sampler) and an inner cone (skimmer). The inter-conal region is evacuated by a rotary pump.
-
(4)
The “hexapole” or “the collision cell”, a highly efficient focussing device for analyte ions which also eliminates argon based polyatomic interferences. Hydrogen and helium gases are circulated within the hexapole.
-
(5)
The mass analyzer comprising of a Quadrupole assembly with range 4–300 AMU.
-
(6)
Vacuum system: comprises of a four stage differentially pumped vacuum system with three turbo pumps, backed by two rotary pumps.
-
(7)
Detector, a selectable gain conversion dynode–scintillator–photomultiplier.
Tuning of ICP-MS
Before carrying out the determinations, initial tuning of the instrument was carried out which involved optimization of various instrumental parameters [gas flows(of both plasma and collision cell), torch positions, voltages etc.] to achieve optimum sensitivity and background levels as well as minimization of levels of doubly charged species and oxide species which lead to isobaric interferences.
Selection of appropriate isotopes of various elements
The isotopes used for measurement were carefully selected to avoid all possible isobaric interferences. The zircon matrix did not affect the elements determined. However, elemental correction equations were also used wherever necessary to correct for inter-REE oxide-element interferences and barium oxide interference on europium isotopes.
Reagents and standards
All solutions were made up in using Grade 1 Millipore water of conductivity of 0.4 μ Siemens. Analytical reagent-grade HF, HCl and HNO3 were used and were purified prior to use by sub-boiling distillation. Standard solutions of various metals of required concentrations were made using very high pure chemicals (99.999%). First, single-element (1,000 μg/mL) stock solutions were prepared using pure metals or pure metal oxides. Mixed multi-element standard solution (10 μg/mL) was prepared from the above stock solution by appropriate dilution. Multi-element working standards containing 1, 10 and 100 ng/mL of each analyte were prepared by successive dilutions. Solutions of certified reference materials from CCRMP: SY-2 and SY-4, prepared in a similar manner as the sample solutions were also used for calibration of rare-earths, uranium and thorium.
Sample dissolution methods
Sodium peroxide fusion method
A 0.1 g sample was accurately weighed into a nickel crucible containing 2 g sodium peroxide, fused till the melt was red hot and clear. The cooled melt was quantitatively transferred into a beaker with 10% (v/v) HCl. Then, concentrated HCl (12 M) was added along with few drops of hydrogen peroxide (30% v/v) and boiled for 5 min to ensure complete dissolution of the fused mass. Then, hydroxide group precipitation was carried out by adding 2 g ammonium chloride and 25% ammonium hydroxide solution. The ammonia group precipitate in the beaker was digested for about 30 min on a water bath and filtered through a Whatman 540 grade filter paper. The precipitate was thoroughly washed with a solution of 5% (v/v) ammonium hydroxide in 1% (w/v) ammonium chloride until it was almost free from nickel denoted by the disappearance of any blue tint. The filtrate was discarded. The precipitate was washed back into the same beaker from the filter paper with a fine jet of water and about 5 mL of concentrated nitric acid and brought to a final volume of 100 mL using Millipore grade water. Procedural blank solutions were also prepared concurrently following the above procedure.
Fluoride fusion method
A 0.1 g sample was mixed with 2 g flux (a homogenous mixture of KHF2 and NaF in 3:1 ratio) in a platinum crucible and fused to red heat over a burner. The crucible was cooled; 10 mL of 18 N sulphuric acid was added and heated on a sand bath till the evolution of sulphuric acid fumes to ensure removal of free fluoride. The contents were cooled, transferred to a beaker with 5 mL of concentrated nitric acid, boiled to get a clear solution and made up to 100 mL volume using Millipore grade water. Procedural blank solutions were also prepared concurrently following the above procedure.
Results and discussion
Choice of sample dissolution methods
Fusion digestion technique has many advantages over the conventional open acid digestion method: as it does not require hazardous HF; dissolves all common rock-forming minerals, including refractory mineral phases; all samples can be processed as a batch through each step; and fast in the sense that a large number of sample solutions can be prepared in a few days. Although LiBO2 fusion is a powerful decomposition technique for geological materials by ICP-MS, this method precludes the determination of volatile elements due to high fusion temperature (1,050 °C). In the present work, despite the introduction of relatively high total dissolved solids (TDS) during sample preparation, both alkali peroxide fusion and fluoride mixture fusion were found to be useful and effective alternative techniques capable of decomposition of zircon samples. In the procedures adopted in the present study, the TDS is extremely low due to the dilution involved and problems like blockage of instrumental parts like cones and nebulizers were not encountered. Procedural blanks were analysed concurrently along with the samples and the counts obtained for the blanks were deducted from those of sample solutions for all isotopes determined. The use of high purity fusion fluxes resulted in extremely low procedural blanks and determination limits did not suffer much when compared to the open acid digestion method.
Determination of REE, hafnium, uranium and thorium in zircon reference materials
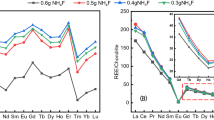
Two zircon reference materials BGS IGS 35 and CERAM 2CAS15 were analysed in the present study by both methods and the results obtained are given in Tables 2 and 3. The values obtained for REE, yttrium and hafnium by both methods were in good agreement with each other. Certified values for the concentration of these elements are not available. However, the concentrations of these elements have been reported by Perkins et al. [9] for IGS 35. The values obtained in the present study agreed well with those reported by Perkins et al. for IGS 35 reference material and also with those obtained after fluoride separation by ICP-AES in the case of REE and thorium values (Tables 2, 3). But no such studies have been reported in literature for 2CAS15 reference sample. Hence, the values reported in the present work can serve as usable values for the above two reference materials.
Determination of REE, hafnium, uranium and thorium in zircon samples
Four zircon samples originating from different areas on the east coast of India were also analysed by the present methods and the results obtained are given in Table 4. The zircon samples show enrichment of the heavy rare-earths and yttrium. The values obtained for rare-earths, yttrium and hafnium by both fusion methods were found to agree well. Relative standard deviation ranging from 1 to 12% was obtained for the studied elements at different concentration levels. The zircon samples were also analysed by ICP-AES after sample dissolution using peroxide fusion followed by ammonia precipitation and fluoride separation. The results obtained (Table 4) compared well with those obtained by ICP-MS.
Conclusions
The proposed fusion methods for the analysis of zircon samples are effective in achieving complete dissolution of zircon samples. The methodology involved is simple and further, the methods do not require the use of any specialized high temperature–high pressure sample dissolution systems and hence, are suitable for a routine analytical laboratory tasked with the analysis of a large number of samples. For the determination of REE, both fusion procedures can be employed. However, the fusion method using fluoride mixture to decompose the zircon samples is simpler and able to provide a fast throughput of data for all required analytes.
References
Williams IS (1992) Trans R Soc Edinb Earth Sci 83:447
Hoskin PWO, Ireland TR (2000) Geology 28:627
Pupin JP (1992) Bull Soc Géol Fr 163:495
Shinjoe H, Orihashi Y, Yoshida HY (2003) Goldschmidt conference Kurashiki Japan abstracts, vol A432
Hoskin PWO (1998) J Trace Microprobe Tech 16:301
Cesbron F, Blanc P, Ohnenstetter D, Rémond G (1995) Scanning Microsc Suppl 9:35
Hanchar JM, Miller CF (1993) Chem Geol 110:1
Zinner E, Crozaz G (1986) Int J Mass Spectrom Ion Proced 69:17
Perkins WT, Pearce NJG, Fuge R (1992) J Anal At Spectrom 7:611
Jarvis KE, Gray AL, Houk RS (1992) Handbook of inductively coupled plasma spectrometry. Blackie, Glasgow
Wu S, Zhao YH, Feng X (1996) J Anal At Spectrom 11:287
Yoshida S, Muramatsu Y, Tagami K, Uchida S (1996) Int J Environ Anal Chem 63:195
Balaram V, Gnaneshwar Rao T (2003) At Spectrosc 24:206
Yu Z, Robinson P, McGoldrick P (2001) Geostand Newsl 25:199
Roy P, Balaram V, Bhattacharaya A, Nasipuri P, Satyanarayanan M (2007) Curr Sci 93(8):1122
Catalogue No. 786a (2010) Outside-source reference materials. Bureau of Analysed Samples Ltd. (BAS)
Acknowledgment
The authors thank Director, Addl. Director (R&D) and Head (Chemistry Group), A.M.D for their encouragement and support in carrying out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.K., Padmasubashini, V. & Gopal, L. Determination of uranium, thorium and rare-earth elements in zircon samples using ICP-MS. J Radioanal Nucl Chem 294, 19–25 (2012). https://doi.org/10.1007/s10967-011-1520-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1520-1