Abstract
A solvent extraction process is proposed to recover uranium and thorium from the crystal waste solutions of zirconium oxychloride. The extraction of iron from hydrochloride medium with P350, the extraction of uranium from hydrochloride with N235, and the extraction of thorium from the mixture solutions of nitric acid and the hydrochloric acid with P350 was investigated. The optimum extraction conditions were evaluated with synthetic solutions by studying the parameters of extractant concentration and acidity. The optimum separation conditions for Fe (III) are recognized as 30% P350 and 4.5 to 6.0 M HCl. The optimum extraction conditions for U (VI) are recognized as 25% N235 and 4.5 to 6.0 M HCl. And the optimum extraction conditions for Th (VI) are recognized as 30% P350 and 2.5 to 3.5 M HNO3 in the mixture solutions. The recovery of uranium and thorium from the crystal waste solutions of zirconium oxychloride was investigated also. The results indicate that the recoveries of uranium and thorium are 92 and 86%, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The energy crisis is one of the three magnitude resource crisis. Uranium and thorium are the most important elements for nuclear energy field. However, both of uranium and thorium are rare metals. In view of increasing demand of the two useful elements in nuclear energy programs, it has to be recovered from industrial waste. Zircon generally contains some amounts of uranium, thorium, scandium, titanium, aluminum, iron, etc. The ore is the most common raw material used for the commercial production of zirconium, such as zirconium oxychloride by alkali fusion method. So, the crystal wastewater of zirconium oxychloride contains sizeable uranium and thorium. With this regard, it remains still a problem both for resource recycle and environmental protection how to recover these nuclear energy elements (uranium and thorium) from the crystal wastewater which is from the processing of zirconium oxychloride.
Solvent extraction is one of the most practiced and cleanest techniques for the separation of uranium and thorium from other associated metal elements. Over the years, various extractants for the tow elements were investigated. The extraction of uranyl nitrate from nitric acid with diisoamyl methylphosphanate (DAMP) was studied [1]. It was shown that the distribution coefficients of U (VI) from nitrate medium with DAMP are considerably higher than with tributyl phosphate (TBP). The extraction of U (VI) with di-2-ethylhexyldithio acid (HEhdtp) from aqueous solutions containing nitrate, chloride, sulfate and phosphate anions was investigated [2]. Senol [3] reported the extraction of U (VI) from aqueous acidic (HCl and HNO3) solutions into a co-existing organic phase containing triisooctyl amine (Alamine 308), tri-n-butyl phosphate (TBP) or bis(2,4,4-trimethylpentyl) monothiophosphinic acid. Karve and Gaur [4] reported that U (VI) was quantitatively extracted from nitric acid with Cyanex 302 (a mixture of trialkylphosphine oxides). The extraction of U (VI) from nitric acid medium with di-isodecyl phosphoric acid (DIDPA) was investigated for a wide range of experimental conditions [5]. Saleh [6] reported the extraction of Th (IV) from nitric acid with TBP. The extraction of thorium sulphate and halides with bis(2-ethylhexyl) phosphoric acid (HDEHP) and tri-n-butyl phosphate (TBP) was investigated [7]. The partition coefficient of Th (IV) is greatly affected by these additives, such as alcohols, acetone and haloacids. Petkovic [8] presented the thermodynamics of extraction equilibrium of thorium nitrate with tri-n-butyl phosphate. The extraction of Th (IV) from perchlorate or nitrate solutions with dialkyldithiophosphoric acids (Hdtp) showed that the chain length in Hdtp significantly influence the magnitude of the distribution ratio [9]. Khan et al. [10] studied the extraction of Th (IV) from nitric acid solution with bis-2-(butoxyethylether). The extraction of Th (IV) from chloride medium with 2-ethyl hexyl phosphonic acid mono-2-ethyl hexyl ester (PC-88A) was investigated [11]. Karve and Gaur [12] reported that Th (IV) was quantitatively extracted from nitric acid medium with using Cyanex 302. The extraction of U (VI) from sulfuric acid solution by long-chain aliphatic amines has been investigated [13]. Studies on the extraction of U (VI) and Th (IV) from sulfuric acid solutions using trilaurylamine (TLA) indicated that the two elements have low distribution coefficients from sulfuric acid solutions and have high distribution coefficients from binary mixture solutions of sulfuric acid and hydrochloric acid or hydrobromic acid [14]. The extraction of U (VI) and Th (IV) from nitric acid and hydrofluoric acid with TBP was studied. Contrary to uranium, the extraction of thorium is much reduced as the concentration of hydrofluoric acid increases [15]. Turanov et al. [16] investigated the extraction of U (VI), Th (IV), and rare-earth elements from nitric acid solutions with phosphoryl- and carbonyl-containing podands. The extraction ability of carboxylic acid amides toward U (VI) is close to that of tributyl phosphate. Extraction of U (VI) and Th (IV) from nitric acid medium with tetraarylsubstituted (o-phenyleneoxymethylene) diphosphine dioxides is studied [17]. Movaseghi [18] reported that U (VI) is separated from iron in nitric acid or ammonium nitrate saturated solutions and from Th (IV) in ammonium nitrate saturated solutions with tributylphosphate (TBP) by solvent extraction. An extraction method was proposed for the extraction of uranium from salicylate media using tris-(2-ethylhexyl) phosphate as an extractant [19]. The method permits the separation of U (VI) from Th (IV), cerium (IV), titanium (IV), zirconium (IV), hafnium (IV), copper (II), vanadium (V) and chromium (VI) from their binary mixtures and is applicable to the analysis of uranium in synthetic samples. Schrotterrova et al. [20] investigated the extraction of zirconium preferentially from U (VI) in sulfuric acid with primary amine Primene JMT (RNH2, R = C19–C22). The behaviour of extraction of zirconium (IV), U (VI), Th (IV), titanium (IV), cerium (III), ytterbium (III), iron (III), aluminum (III), and manganese (II) from mineral acid (sulfuric acid, nitric acid and hydrochloric acid) using Cyanex 923 (a mixture of trialkylphosphine oxides) revealed that zirconium (IV) can be separated from U (IV) and Th (IV) at 0.5 mol/L H2SO4 [21]. The extractive separation of U (VI) and Th (IV) in presence of titanium, iron and lanthanum in nitrate medium was investigated [22]. A process containing TBP-Cyanex 302 (the first stage using TBP and the second stage using Cyanex 302) was proposed for separation uranium and thorium from Zarigan ore leach solution with nitric acid.
Amongst the extractants for the extraction of uranium and thorium, the organophosphorous compounds have found wide application. Though many reports are on the extraction of uranium and thorium, most of medium given in the reports is nitric acid solutions. However, there are no reports to investigate the recovery of uranium and thorium from the crystal waste solutions of zirconium oxychloride by solvent extraction so far. In addition, the crystal wastewater contains zirconium, iron, uranium, thorium, scandium, titanium, and aluminum. So, the extraction system to recovery the nuclear energy elements from the crystal wastewater is quite complex.
This article presents an effective process for the recovery of uranium and thorium from crystal wastewater zirconium oxychloride by solvent extraction. First, the iron (III) is preferentially extracted away from original crystal wastewater, hydrochloride solutions, with di(1-methyl heptyl) methyl phosphate (commercially known as P350). And then, the U (VI) is selectively recovered from hydrochloride solution (the raffinates after extraction with P350 early) using tertiary amine N235 (mixture of tertiary amine R3N, R = C8–C10, molecular weight 350–390). At last, Th (IV) is separated from the mixture medium of nitric acid and hydrochloric acid with P350.
Experimental
N235, P350, and 2-octanol were supplied by Shanghai Rare-Earth Chemical Co., Ltd., China. N235 and 2-octanol were used without further purification. P350 was purified by washing with 10% Na2CO3. Sulphonated kerosene (used as diluents) was obtained from Zibo Qining Fine Chemical CO., Ltd., China. Crystal waste solutions of zirconium oxychloride were received from Jiangxi Kingan Hi-Tech Co., Ltd., China. The other chemicals used in this work were of analytical reagent grade. The stock solutions (containing appropriate amount of hydrochloric acid to prevent hydrolysis of metal ions) of each metal ion were prepared by dissolving relevant oxide in hydrochloric acid or prepared by dissolving relevant chloride in distilled water, respectively.
The extraction experiments were performed mechanically in a separatory funnel at room temperature with the phase volume ratio O/A fixed at 1:1. The organic solutions of P350 were prepared by dissolving P350 in sulphonated kerosene. The organic solutions of N235 were prepared by mixing N235 and 2-octanol with sulphonated kerosene, and the 2-octanol concentration in the organic solutions was fixed at 10% (v/v) unchangeably. The aqueous solutions were synthetic solutions containing appointed metal ion concentrations or crystal waste solutions of zirconium oxychloride. The extraction experiments with N235 were carried for 5 min, and the experiments with P350 for 15 min. The organic and aqueous phases were allowed to settle and then separated, and a suitable aliquot of the aqueous raffinate was analyzed by sequential inductively coupled plasma spectrometer (JY-38 PLUS, France). The metal ion concentration in stock solutions was determined by titration, and the concentration in loaded organic phase was calculated by mass balance, respectively. The H+ ion concentration was determined by acid base titration with a standard NaOH solution using methyl red as an indicator.
Result and discussion
Extraction of iron from hydrochloric acid medium with P350
The P350 concentration in sulphonated kerosene as the diluent has been investigated in the range of 10 to 40% (v/v). The effect of P350 concentration on the extraction of appointed metal ions from synthetic solutions containing 5.0 M HCl by equal volumes of aqueous phase and organic phase is shown in Fig. 1. It is evident from Fig. 1 that Fe (III) is preferentially extracted away from the associated metal ions. The extraction of Fe (III) increases rapidly with P350 concentration in organic phase from 10 to 30%, and approaches almost 100% beyond 30% P350. As P350 concentration increases from 10 to 40% P350, the extraction of U (VI), Th (IV), and Sc (III) increases very slowly, and the extraction of the three metal ions is lower than 10% on the whole. Furthermore, the extraction of Zr (IV), Ti (IV), Y (III), and Al (III) is negligible in the entire investigated range of P350 concentration.
The effect of hydrochloric acid concentration on the extraction of appointed metal ions from synthetic solutions with 30% (v/v) P350 is illustrated in Fig. 2. Figure 2 indicates that the hydrochloric acid concentration is a important parameter in the extraction of Fe (III). The extraction of Fe (III) increases slowly with the hydrochloric acid concentration from 1.0 to 2.5 M first, and increases rapidly with the hydrochloric acid concentration from 2.5 to 4.0 M then, and reaches almost 100% beyond 4.0 M hydrochloric acid last. As hydrochloric acid concentration rises from 1.0 to 6.0 M, the extraction of U (VI), Sc (III),and Th (IV) is all lower than 10%, and the extraction of Zr (IV), Ti (IV), Y(III), and Al (III) is negligible.
From Figs. 1 and 2, the optimum separation conditions for Fe (III) from synthetic solutions containing the given metal ions are recognized as 30% P350 and 4.5 to 6.0 M HCl.
Extraction of uranium from hydrochloric acid medium with N235
The phase volume ratio O/A and the 2-octanol concentration in the organic solutions have been kept at 1:1 and 10% (v/v), respectively. The effect of N235 concentration on the extraction of appointed metal ions from synthetic solutions containing 5.0 M HCl is plotted in Fig. 3. From the plots it is apparent that the U (VI) is selectively extracted from hydrochloride solution containing appointed metal ions using N235. The extraction of U (VI) increases slowly from 10 to 25% N235, and then remains about 100% beyond 25% P350. The extraction of Th (IV), Zr (IV), Ti (IV), Y (III), Sc (III), and Al (III) with N235 at 5.0 M HCl is insignificant.
The effect of hydrochloric acid concentration on the extraction of appointed metal ions from synthetic solutions with 25% (v/v) N235 and 10% (v/v) 2-octanol in sulphonated kerosene is given in Fig. 4. As can be seen, the extraction of U (VI) increases with hydrochloric acid concentration from 1.0 to 4.0 M, and then approaches 100% beyond 4.0 M HCl. The extraction of Th (IV), Zr (IV), Ti (IV), Y (III), Sc (III), and Al (III) with N235 is insignificant in the range of 1.0 to 6.0 M HCl.
From Figs. 3 and 4, the optimum extraction conditions for U (VI) from synthetic solutions containing the given metal ions are recognized as 25% N235 and 4.5 to 6.0 M HCl.
Extraction of thorium from mixture of nitric acid and hydrochloric acid with P350
The phase volume ratio O/A and hydrochloric acid concentration in the synthetic solutions have been maintained at 1:1 and 5.0 M, respectively. The effect of P350 concentration on the extraction of appointed metal ions from synthetic solutions (mixture of nitric acid and hydrochloric acid) containing 3.0 M HNO3 is presented in Fig. 5. As indicated in Fig. 5, the extraction of Th (IV) rises rapidly from 10% P350 to 30% P350, and rises rather slowly beyond 30% P350. As P350 concentration in organic phase increases from 10 to 40%, the extraction of Sc (III) and Zr (IV) increases a little, and is lower than 10% on the whole. Moreover, the extraction of Ti (IV), Y (III), and Al (III) is ignorable in the range of 10–40% P350.
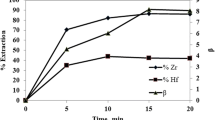
The effect of nitric acid concentration on the extraction of appointed metal ions from synthetic solutions containing 5.0 M HCl with 30% (v/v) P350 in sulphonated kerosene is shown in Fig. 6. From Fig. 6, the extraction of Th (IV) increases quite sharply from 1.0 to 2.5 M HNO3, and reaches the maximum at 3.5 M HNO3, and decreases a little beyond 3.5 M HNO3. As nitric acid concentration increases from 1.0 to 5.0 M, the extraction of Sc (III) and Zr (IV) rises slightly, and is all lower than 10%. Moreover, the extraction of Ti (IV), Y (III), and Al (III) is ignorable in the entire investigated range of nitric acid molarity.
From Figs. 5 and 6, the optimum recovery conditions for Th (VI) from synthetic solutions containing the given metal ions are recognized as 30% P350 and 2.5 to 3.5 M HNO3.
Recovery process
The recovery process consists of three steps. The first step is to extract iron (III) from the crystal waste solutions of zirconium oxychloride with 30% P350. Iron (III) is preferentially extracted into to P350 organic phase, and the raffinate after extraction of iron with P350 is used as the feed solution to recover uranium next step. The second step is to extract U (VI) with 25% N235 from the raffinate after extraction of iron previously. U (VI) is selectively extracted into the N235 organic phase, and the raffinate after extraction of uranium with N235 is used as the raw material solution to recover thorium last step. The last step is to extract thorium with 30% P350 from the mixture solutions of nitric acid and the raffinate after extraction of uranium. The mixture solutions for extraction of thorium prepared by adding 23.1 mL concentrated nitric acid (to control the mixture solutions containing about 3.0 M HNO3) into 100 mL raffinate after extraction of uranium with 25% N235.
First, the extraction of iron from the crystal waste solutions of zirconium oxychloride with 30% P350 has been investigated. The crystal waste solutions contain 5.1 M HCl. The chemical composition of the crystal waste solutions as received and the extraction results are presented in Table 1. Table 1 shows the extraction of iron is 99.3%, and the extraction of other said metal ions is lower than 10%.
Where, c f is the concentration in the feed solutions before extraction, c r is the concentration in the raffinate after extraction, and E is the extraction.
And then, the extraction of uranium with 25% N235 from the raffinate after extraction of iron has studied. The extraction results are presented in Table 2. Table 2 shows the extraction of uranium is 99.5%, and the extraction of other said metal ions is lower than 5%. From Tables 1 and 2, the recovery of uranium is about 92%.
The extraction of thorium with 30% P350 from the mixture solutions of nitric acid and the raffinate after extraction of uranium has studied. The chemical composition of the mixture solutions and the extraction results are presented in Table 3. Table 3 shows the extraction of uranium is 95.2%, and the extraction of other said metal ions is lower than 10%. From Tables 1 and 3, the recovery of thorium is about 86%.
Conclusion
The present investigations were developed a convenient and effective method for the recovery of uranium and thorium from the crystal waste solutions of zirconium oxychloride. The process consists of (a) the extraction of iron from the crystal waste solutions of zirconium oxychloride with 30% P350; (b) the extraction of uranium with 25% N235 from the raffinate after extraction of iron; and (c) the extraction of thorium with 30% P350 from the mixture solutions of nitric acid and the raffinate after extraction of uranium. The recoveries of uranium and thorium are 92 and 86%, respectively.
References
Shevchenko VB, Slepchenko IG, Shmidt VS, Nenarokomov EA (1961) Extraction properties of diisoamyl methylphosphinite. Atom Energy 7:728–734
Haiduc I, Curtui M, Haiduc I (1986) Solvent extraction of uranium (VI) with di-2-ethylhexyldithiophosphoric acid from aqueous nitrate, chloride, sulfate and phosphate medium. J Radioanal Nucl Chem 99:257–263
Senol A (2003) Liquid–liquid extraction of uranium(VI) from aqueous acidic solutions using alamine, TBP and CYANEX systems. J Radioanal Nucl Chem 258:361–372
Karve M, Gaur C (2007) Extraction of U(VI) with Cyanex 302. J Radioanal Nucl Chem 273:405–409
Biswas S, Singh DK, Hareendran KN, Sharma JN, Roy SB (2010) Extraction behavior of U (IV) from nitric acid medium using di-isodecyl phosphoric acid dissolved in dodecane. J Radioanal Nucl Chem 284:201–205
Saleh FA (1969) Effect of diluents on the extraction of thorium nitrate with tributyl phosphate. Zeitschrift für anorganische und allgemeine Chemie 371:106–112
Haggag A, El-fekey SA, Yehia EM, Sanad W (1976) J Radioanal Nucl Chem 33:139–152
Petkovic DM (1988) Solvent extraction of thorium nitrate with tri-n-butyl phosphate. J Chem Soc Dalton Trans 7:1813–1816
Curtui M, Haiduc I, Haiduc I (1992) Solvent extraction of thorium (IV) with dialkyldithiophosphoric acids. J Radioanal Nucl Chem 165:95–105
Khan M, Ali A (1996) Extraction of thorium from aqueous nitric acid solution by bis-2-(butoxyethyl ether). J Radioanal Nucl Chem 203:161–167
Singh DK, Singh H, Gupta CK, Mathur JN (2001) Extraction of thorium with 2-ethyl hexyl phosphonic acid mono-2- ethyl hexyl ester. J Radioanal Nucl Chem 250:123–128
Karve M, Gaur C (2006) Liquid–liquid extraction of Th (IV) with Cyanex 302. J Radioanal Nucl Chem 270:461–464
Sato T (1968) The extraction of uranium from sulphuric acid solutions by long-chain aliphatic amines. J Inorg Nucl Chem 30:1065–1074
Souka N, Shabana R, Hafez F (1975) Extraction behavior of thorium, protactinium, uranium and neptunium from mixed mineral acid solution by TLA. J Radioanal Nucl Chem 27:401–410
Volk VI, Vakhrushin AY, Mamaev SL (2000) Extraction of uranium and thorium with TBP from fluoride–nitric acid solutions. J Radioanal Nucl Chem 246:697–702
Turanov AN, Karandashev VK, Baulin VE (2007) Extraction of U(VI), Th(IV), and rare-earth elements from nitric acid solutions with phosphoryl- and carbonyl-containing podands. Radiochemistry 49:256–263
Turanov AN, Karandashev VK, Yarkevich AN, Baulin VE (2009) Extraction of U(VI) and Th(IV) from nitric acid solutions with tetraaryl-substituted (o-phenyleneoxymethylene) diphosphine dioxides. Radiochemistry 51:359–364
Movaseghi H (1986) Method of separating uranium from iron and thorium. J Radioanal Nucl Chem 99:331–335
Sundaramurthi NM, Desai GS, Shinde VM (1990) Extraction and separation studies of uranium (VI) with tris-(2-ethyl hexyl) phosphate. J Radioanal Nucl Chem 144:439–445
Schrotterova D, Nekovar P, Mrnka M, Bousa M (1992) Application of amine extraction to the production of pure zirconium salts. J Radioanal Nucl Chem 163:29–36
Gupta B, Malik P, Mudhar N (2005) Extraction and recovery of zirconium from zircon using Cyanex 923. Sol Extr Ion Exch 23:345–357
Nasab ME, Milani SA, Sam A (2011) Extractive separation of Th(IV), U(VI), Ti(IV), La(III) and Fe(III) from Zarigan ore. J Radioanal Nucl Chem 288:677–683
Acknowledgments
This research was supported financially by Scientific and Technical Research Foundation of Education Ministry of Jiangxi Province (No. DB201002119), and Scientific and Technical Research Foundation of Nanchang Hangkong University (No. EA200902030).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, X., Wu, Y. Recovery of uranium and thorium from zirconium oxychloride by solvent extraction. J Radioanal Nucl Chem 292, 355–360 (2012). https://doi.org/10.1007/s10967-011-1409-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1409-z