Abstract
Liquid–liquid extraction of U (VI) from concentrated phosphoric acid by using (2-ethyl hexyl) phosphonic acid, mono (2-ethyl hexyl) ester (PC88A) and di-butyl butyl phosphonate (DBBP) has been investigated. The effect of different factors affecting the extraction process (PC88A concentration, DBBP concentration, shaking time, aqueous/organic phase ratio, phosphoric acid concentration and effect of diluents) have been investigated. The obtained data of temperature on the extraction showed that the enthalpy change is −17.15 kJ mol−1. Uranium was extracted from the strip liquor by using di (2-ethylhexyl) phosphoric acid and tri-octyl phosphine oxide mixture and finally converted to a high purity UO3 product using precipitation with hydrogen peroxide and heat treatment at 365 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is an element of great commercial interest because of its use in the production of nuclear energy, in the manufacture of nuclear weapons [1]. Natural phosphates are found to contain several tens to hundreds of parts per million of uranium depending upon the origin of the phosphate rocks [2]. Due to its radioactivity and toxicity (carcinogenic for humans) [3], rock phosphates which is used for the production of Wet process phosphoric acid (WPPA) considered as secondary source to uranium and rare earth production [4, 5], hence the phosphoric acid product may contain up to 300 ppm uranium together with other rare metals such as V, Cd, and Co and radionuclides like Th and Ra [6, 7].
Several processes have been developed for the recovery of uranium from phosphoric rock like solvent extraction, ion exchange, precipitation etc. The solvent extraction method has been one of important techniques in concentrating and purification of uranium [8]. Solvent extraction processes using organophosphorus reagents have been developed for uranium separation from process streams including phosphoric acid as well as partially neutralized phosphoric acid [9].
Among these systems, 2-ethyl hexyl phosphoric acid-mono-2-ethyl hexyl ester (PC88A) has been used for several decades as the most successful extractant for recovery of uranium from phosphoric acid, which has higher steric hindrance and higher selectivities than di(2-ethylhexyl)phosphoric acid (D2EHPA) [10]. Studies on the use of di-butyl butyl phosphonate (DBBP) as a synergistic agent, for uranium separation from phosphoric acid were carried out [11].
In the present study, uranium extraction from WPPA was investigated using PC88A, either alone or its mixture with DBBP in kerosene as extractants. The effects of various experimental factors are investigated to optimize the suitable conditions for separation of uranium from phosphoric acid. Kinetic studies for the uranium extraction from phosphoric acid by using combination of PC88A and DBBP system includes enthalpy, entropy and activation energy was investigated.
Experimental
Reagent
In the present studies WPA was obtained from Abu-Zaabal Company, Egypt. The acid must be treated to separate humic matter before extraction tests. PC88A, (purity > 95%) chemically known as 2-ethyl hexyl phosphoric acid-mono-2-ethyl hexyl ester supplied by Daihachi Chemical Industry Co. Ltd. Japan was used as supplied. (DBBP), (purity 95%) which supplied by Fluka and kerosene as a diluent obtained from MISR-Petroleum Ltd. Company, Egypt.
Analysis
Uranium concentrations in WPA sample as well as in other aqueous streams, generated during batch experiments, were analyzed by the Arsenazo III method [12]. Absorbance of the formed uranium Arsenazo III complex was measured at 650 nm against proper standard solutions. For this purpose, a Lambada3 UV/Vis spectrophotometer (Perkin-Elmer, USA) was used.
Extraction procedure
Egyptian commercial phosphoric acid containing 62 mg L−1 uranium was used in this extraction studies, the chemical composition of this phosphoric acid is shown in Table (1). In these studies, a fixed volume of the aqueous phase (25 mL) was used and the distribution of uranium in aqueous/organic phase was expressed in percentage or distribution coefficients using standard method. Distribution ratio was calculated using Eq. (1) at equilibrium. The extraction kinetics has been studied using separator funnels, by determining the variation of extraction coefficient as a function of time for various surfactant and co-extractant concentrations. The effects of various parameters, such as concentrations of organic solvent, phosphoric acid, shaking time, and temperature, on the extraction kinetics were studied.
where: D u: uranium distribution ratio, C org: concentration of uranium in organic phase, C aq: concentration of uranium in aqueous phase.
Results and discussion
Effect of phosphoric acid concentration on uranium extraction
The effect of phosphoric acid concentration on uranium extraction from phosphoric acid by using 0.8 M PC88A + 0.5 M DBBP/kerosene at A/O = 2 and at room temperature was studied. From the obtained data plotted in Fig. 1, it can be shown that uranium distribution ratio (D u) decrease with increasing the concentration of phosphoric acid. The plot of log D u against log phosphoric acid is shown in Fig. (2), the linear relationship with slope of ~−2 indicates that 2 mol of acid are liberated for extraction of 1 mol of uranium. Based on the above findings, a plausible extraction equilibrium, ignoring the complexation by phosphate ions UO2(H2PO 2−n4 ) n , is postulated as (HX is PC88A):
Effect of PC88A and DBBP concentration on uranium extraction
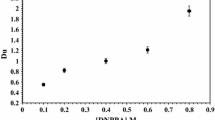
The effect of PC88A and DBBP concentrations upon selective uranium extraction efficiency from the Egyptian concentrated wet process phosphoric acid was studied, a series of extraction experiments was performed using PC88A and DBBP/Kerosene in various concentrations (0.1–1.0 M) and (0.1–0.6 M) for PC88A and DBBP, respectively. In these experiments, the other extraction conditions were fixed at an O/A ratio of 1/1 and using 30 min shaking time at room temperature. From the obtained results shown in Fig. 3 it is clearly obvious that the uranium distribution coefficient increase with increasing initial PC88A and DBBP concentrations followed by slight increase.
Effect of DBBP concentration on uranium extraction at constant PC88A
The synergistic effect of DBBP concentration on the extraction percent of uranium from concentrated phosphoric acid has been investigated. A set of experiments were performed by shaking the treated phosphoric acid with DBBP having concentration ranging from (0.1 to 0.6 M) at constant PC88A concentration (0.8 M) and in O/A phase ratio equal 1.0 for 30 min at room temperature. The obtained results as shown in Fig. (4) indicate that, the uranium distribution ratio decreased up to 0.2 M concentration of DBBP, which means that up to a certain concentration, DBBP is acting as antagonistic agent rather than synergistic agent then a significant improvement in D u start to occur which indicate the occurrence of synergism with increasing DBBP concentration up to 0.5 M.
A plot of log D u versus log [DBBP] at constant PC88A concentration of 0.8 M is presented graphically in Fig. 5 shows a slope of ~1, which indicates that 1 mol of uranium in organic phase is associated with 1 mol of DBBP.
Effect of PC88A concentration on uranium extraction at constant DBBP
The effect of PC88A concentration on the extraction of uranium from phosphoric acid solution at constant 0.5 M DBBP concentration was investigated; Equal volumes (25 mL) of PC88A and 0.5 M DBBP/kerosene and 9.2 M phosphoric acid were mixed together. The results represented in Fig. 6 shows that the uranium distribution ratio (D u) increases with increasing the PC88A concentration. The plot of log D u versus log [PC88A] is shown in Fig. 7 and indicates a linear relationship with slope ~1.
Effect of shaking time on uranium extraction
The shaking time effect upon uranium extraction efficiency from concentrated Egyptian phosphoric acid, 9.2 M, by 0.8 M PC88A + 0.5 M DBBP/kerosene was studied by investigating another series of extraction experiments using different shaking times ranging from 1 up to 30 min. In these experiments, the other extraction conditions were fixed at an O/A = 1, T = 25 ± 1 °C for various time intervals. Figure 8 shows the variation of uranium distribution ratio (D u) against time, it is clear that 15 min is the minimum time to reach the equilibrium.
Effect of aqueous to organic phase ratio on uranium extraction
The effect of the A/O phase ratio on the extraction of uranium was investigated at A/O phase ratio varying from 1.0 to 4.0. All experiments were performed using 0.8 M PC88A + 0.5 M DBBP mixture/kerosene for shaking time of 15 min and at room temperature. The data is shown in Table 2, it is clear that by increasing A/O phase ratio the uranium extraction percent was decreased.
Effect of temperature on uranium extraction
The effect of temperature on the uranium extraction from concentrated phosphoric acid at different temperature was investigated. The extraction experiments were carried out by contacting phosphoric acid with a 0.8 M PC88A + 0.5 M DBBP/kerosene for 15 min while the A/O phase ratio was fixed 2 but the temperature was varied. Figure (9) show the relation between temperature and uranium distribution ratio (D u). From the obtained data, it can be noticed that D u is decreased by increasing the temperature which demonstrates the exothermic nature of the extraction process. The temperature effect up on the distribution coefficient can be quantified by making use of the Van’t Hoff equation, which relates the chemical equilibrium constant with temperature:
By integration,
And since the distribution ratio D is related by definition to the equilibrium constant K the previous equation can be written as:
The plot of log D u against 1/T yields a straight line equation with slope (x) = −∆H°/2.303 R. Figure 10 shows that extraction of uranium by 0.8 M PC88A + 0.5 M DBBP from wet process phosphoric acid decrease with temperature. An enthalpy change of −17.15 kJ mol−1 was determined by using Eq. 5 in the given range of temperature, which indicates that the extraction is an exothermic process.
Effect of diluents
The effect of diluents is shown in Fig. 11, from the obtained data it is clear that, diluents affect the extraction of uranium.
Stripping of uranium from organic solvent
The uranium stripping process by using different concentration of analytical grade phosphoric acid from the synergistic 0.8 M PC88A + 0.5 M DBBP/kerosene at room temperature were investigated. Figure 12 show the relation between phosphoric acid concentrations and the stripping efficiency. It is clear that uranium can be stripped from loaded organic with 11 M analytical grade phosphoric acid containing 10 g/l Fe+2 to reduce the less stripped uranium form (VI) to the more stripped uranium form (IV). other parameters that affected in uranium stripping process were investigated, it was found that, the preferred stripping results are; temperature: 60–70 °C; shaking time: 5 min; O/A phase ratio is equal 18 and five stages were sufficient for stripping about 98% of total uranium in loaded organic.
Uranium recovery
The recovery of uranium from the strip solution can be achieved by processed the strip solution in a second cycle of extraction-stripping with an additional scrubbing step by sulfuric acid incorporated to obtain a uranium cake of high purity. The organic solvent used in the second cycle was 0.3 M D2EHPA + 0.075 M TOPO [13]. Stripping of uranium from the loaded organic phase can be carried out with 1 M ammonium carbonate solution, followed by filtration of the strip liquor in order to remove traces of iron precipitate. Precipitation was carried out using hydrogen peroxide to bring down the pH of the solution, and the neutralization was carried out with sulphuric acid. In a pH range of 3–4, the uranium precipitation was complete (≈99%) and uranium peroxide hydrate was filtered, washed, dried and calcined at 365 °C to obtain UO3 powder with high purity. Figure 13 show flowsheet describe the overall process for uranium recovery from WPPA by using 0.8 M PC88A + 0.5 M DBBP mixture in kerosene.
Conclusion
Uranium can be extracted from wet process phosphoric acid by using 0.8 M PC88A + 0.5 M DBBP in kerosene at room temperature for shaking time 15 min. Chemical mechanism for the uranium extraction process has been postulated based on the results of slope analysis. The studies of the effect of diluents clearly indicate a role of diluent in extraction of uranium from aqueous solutions. Second cycle of extraction from the strip solution was carried out by using 0.3 M D2EHPA + 0.075 M TOPO mixture where in scrubbing step has been incorporated and stripping is performed by an alkaline solution in order to obtain high purity uranium. The precipitation process yields high purity uranium peroxide which is filtered, washed, dried and calcined at 365 °C.
References
Santos JS, Teixeira LSG, dos Santos WNL, Lemos VA, Godoy JM, Ferreira SLC (2010) Uranium determination using atomic spectrometric techniques: an overview. Anal Chim Acta 674:143–156
Ninger RD (2000) Uranium exploration policy, economics and future prospective, IAEA-PL-490/7, US Atomic Energy Commission, USA
Regenspurg S, Schild D, Scafer T, Huber F, Malmstrom ME (2009) Removal of uranium(VI) from the aqueous phase by iron(II) minerals in presence of bicarbonate. Appl Geochem 24:1617–1625
Nagaphani Kumar B, Radhika S, Ramachandra Reddy B (2010) Solid–liquid extraction of heavy rare-earths from phosphoric acid solutions using tulsion CH-96 and T-PAR resins. Chem Eng J 160:138–144
Radhika S, Nagaphani Kumar B, Laskshmi Kanta M, Ramachandra Reddy B (2010) Liquid-liquid extraction, separation possibilities of heavy, light rare-earths from phosphoric acid solutions with acidic organophosphorus reagents. Sep Purif Technol 75:295–302
Scholten LC, Timmermans CWM (1996) Natural radioactivity in phosphate fertilizers. Fertil Res 43:103–107
Jila Q, Tong S, Li Z, Zhou W, Li H, Meng S (2009) Solvent extraction of rare earth elements with mixtures of sec-octylphenoxyacetic acid and bis (2,4,4-trimethylpentyl) dithiophosphinic acid. Sep Purif Technol 64:345–350
Yang Y-Z, Sun S-X, Feng S-Y (2002) Liquid–liquid extraction of uranium (VI) with 2-ethylhexyltolylsulfoxide (EHTSO). J Radioanal Nucl Chem 251(3):503–506
Singh H, Mishra SL, Anitha M, Giriyalkar AB, Vijayalakshmi R, Kotekar MK (2003) Uranium extraction from partially neutralised and diluted acid using a synergistic organophosphorous solvent mixture. Hydrometallurgy 70(1–3):197–203
Wang ZH, Ma GX, Lu J, Liao WP, Li DQ (2002) Separation of heavy rare earth elements with extraction resin containing, 1-hexyl-4-ethyloctyl isopropylphosphonic acid. Hydrometallurgy 66:95–99
Abdel magied AF, Abdel-Khalek AA, Ali MM, Hussein AEM (2011) Liquid–liquid extraction of uranium from Egyptian phosphoric acid using a synergistic D2EHPA–DBBP mixture. J Radioanal Nucl Chem 288:1–7
Marczenko Z (1986) Spectrophotometric determination of the elements. Wiley, New York
Singh H, Vijaylakshmi R, Mishra SL, Gupta CK (2001) Studies on uranium extraction from phosphoric acid using di-nonyl phenyl phosphoric acid based synergistic mixtures. Hydrometallurgy 59:67–76
Acknowledgment
The authors wish to thank Dr. H. S. Gado Head of phosphoric acid pilot plant for his valuable help in carrying out the test work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Khalek, A.A., Ali, M.M., Ashour, R.M. et al. Chemical studies on uranium extraction from concentrated phosphoric acid by using PC88A and DBBP mixture. J Radioanal Nucl Chem 290, 353–359 (2011). https://doi.org/10.1007/s10967-011-1372-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1372-8