Abstract
In vivo imaging of tumours using radiolabelled somatostatin (SST) analogues has become an accepted clinical tool in oncology. HYNIC-Tyr3 octreotide and Tyr3 octreotide were synthesized by FMOC solid-phase peptide synthesis using a semi-automated synthesizer. These were analyzed and purified by RP-HPLC, mass spectroscopy, IR spectroscopy, 1H NMR and 13C NMR. The prochelator 6-BOC-HYNIC was also synthesised and characterised indigenously. HYNIC-Tyr3 octreotide was labelled with 99mTc using Tricine and EDDA as coligand by SnCl2 method. Labelling with 99mTc was performed at 100 °C for 15 min and radiochemical analysis by ITLC and HPLC methods. The radiochemical purity of the complex was over 98% and log p value was found to be −1.27 ± 0.12. The stability of radiolabelled peptide complex was checked at 37 °C up to 24 h. Blood clearance and protein-binding study was also performed. In vivo biodistribution studies in rat showed uptake of 99mTc-HYNIC-TOC in kidney than any other organs. The blood clearance was faster with rapid excretion through kidneys and relatively low uptake in liver.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vivo imaging of tumours using radiolabelled somatostatin (SST) analogues has become an accepted clinical tool in oncology [1]. Radiolabeled peptides are currently under investigation in nuclear oncology for tumour diagnosis and therapy. The first radiopharmaceutical of choice for this diagnostic procedure is [111In-DTPA-DPhe1]-octreotide (111In-DTPA-octreotide) [2]. But the use of 111In as a radioisotope results in high cost, long half life (67 h), suboptimal image quality and a high radiation burden to the patient [2]. So these disadvantages could be overcome by the use of 99mTc labelled somatostatin analogs. 99mTc (6 h half life and 140 keV monoenergetic gamma ray emission) has been considered as the radionuclide of choice with ease of availability, ideal for SPECT-Nuclear Medicine imaging procedures [3]. Gandomkar et al. has synthesized HYNIC-TOC and HYNIC-TATE in solid phase using N-hydroxybenzotriazole (HOBt) as coupling agent. But they have cyclised the peptide chain by formation of cys–cys disulfide bond in solution phase through treatment with 10 eq iodine for 30 min [5]. Storch et al. has synthesized linear peptide chain of Tyr3-octreotide and Tyr3-octreotate in solid phase using HOBt as coupling agent and coupling of BOC-HYNIC was done in solution phase by using O-(7-azabenzotriazol-1-yl)-1,1,3,3 tetramethyluronium hexafluorophosphate (HATU). Schottelius et al. has synthesized DOTA-Tyr3-octreotide and DOTA-Tyr3-octreotate. Cyclisation was performed in THF containing 5–10% of 5 mM ammonium acetate. The solution was buffered to pH = 7 with saturated NaHCO3 and H2O2 in solution phase [8]. Lee et al. has synthesized Tyr3-octreotide in solid phase using FMOC-amide resin and HBTU/HOBT as coupling agent. They have cyclised the peptide chain by formation of cys–cys disulfide bond in solid phase through treatment with 10 eq iodine for 60 min.
Our aim is to formulate in-house kit for ready to use for preparation of 99mTc-HYNIC-Tyr3-octreotide radiopharmaceutical by following an indigenous method. The solid-phase synthesis of HYNIC-Tyr3-octreotide, radiolabelling and biological evaluation of the labelled octreotide are described in this report. We have tried to synthesize HYNIC-TOC and cyclised the peptide chain by formation of cys–cys disulfide bond in solid phase [9].
Experimental
Materials and methods
All chemicals and solvents were of either HPLC or analytical grade and were used without further purification. O-t-butylthreoninol-2-chloro-trityl resin, FMOC-D-Phe, FMOC-Cys (ACM), FMOC-Lys (BOC)-OH, FMOC-D-Trp (BOC)-OH, FMOC-Thr (tbu)-OH, FMOC-Tyr (tBu)-OH and TBTU (2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate), were obtained from NovaBiochem (Switzerland). Di-isopropyl ethylamine (DIPEA) was obtained from SRL, India. SnCl2·2H2O, picryl sulfonic acid, thioanisole and 1,2-ethanedithiol (EDT), trifluoroacetic acid (TFA) were obtained from Sigma Chemicals. All other chemicals and solvents were either obtained from MERCK, Germany or from SRL, India.
13C spectra were recorded on Bruker AVANCE-600 spectrometers (600 MHz) and 1HNMR spectra were recorded on Bruker AVANCE-600 spectrometers (600 MHz). NMR data were reported as follows: chemical shifts in parts per million (ppm) on the δ scale, J values were given in Hz and multiplicity was quoted as follows: s, singlet; d, doublet; t, triplet; dd, doublet of doublets; dt, doublet of triplets; q, quartet; br, broad; m, multiplet; etc. IR spectra were obtained using JASCO FT/IR model 410 instruments. Mass spectrum was recorded on an applied biosystems 4700 proteomics analyzer 170. Quantitative gamma counting was performed on a (Gamma ray spectrometer: GRS 23C, ECIL) well counter.
Solid Phase Peptide synthesis (SPPS)
Tyr3-octreotide (TOC) and HYNIC-Tyr3-octreotide (HYNIC-TOC) were semi-automatically synthesized on a peptide synthesizer (PS3 Protein Technology Instruments, NULAB, USA) followed by standard FMOC solid phase synthesis (SPPS) [3] on O-t-butyl-threoninol-2-chlorotrityl resin (substitution 0.67 mmol/g). Coupling of each amino acid was performed in the presence of 10 mol excess of FMOC-amino acid, 9.5 mol excess 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate) (TBTU), and 20 mol excess of diisopropylethylamine (DIPEA) in DMF. Amino acids were coupled by removing FMOC protecting groups with 20% piperidine/DMF for 10 min followed by washing with DMF. Progress of the amino acid coupling was monitored by color change of picryl sulfonic acid (TNBS). This process was repeated until the required sequence was achieved. Finally for HYNIC-TOC, coupling of HYNIC to peptide was performed in the presence of 5 mol excess of HYNIC-BOC, 4.5 mol excess of 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate) (TBTU) and 10 mol excess of diisopropylethylamine (DIPEA) in DMF for 40 min. Coupling success was checked by TNBS test. The coupled resin was filtered and rinsed with DMF.
Disulfide bond formation
Peptide chain was cycled by formation of cys–cys disulfide bond in solid phase [3] through treatment with 10 eq iodine for 1.30 h at 25 °C.
For deblocking
The S–S cyclised peptide chain with all protecting groups was cleaved after treatment with 95% TFA, 2.5% EDT (ethane dithiol), 2.5% water at 25 °C for 3–4 h. Then it was rinsed with DMF and the crude product was precipitated with cold diethyl ether.
Purification of TOC and HYNIC-TOC
Finally the TOC and HYNIC-TOC were purified and analysed on a Schimadzu HPLC, consisting of two LC20AD solvent pumps and a SPD-M20A prominenance diode array detector. Injections of 150 μL were eluted at a flow rate of 1 mL/min from a μ bondapack C-18, 10 μm, 125 A0, 7.8 × 300 mm reverse phase column, monitored at 254 nm wave length. The gradient systems consisted of 0.1% trifluoroacetic acid/water (solvent A) and 0.085% trifluoroacetic acid/acetonitrile (solvent B) was used. A 32 min binary gradient was used. Gradient I: 0 min 100% A (0% B), 5 min 80% A (20% B), 10 min 65% A (35% B), 15 min 50% A (50% B), 25 min 20% A (80% B), 30 min 0% A (100% B), 32 min 100% A (0% B). The major peak at a retention time of 21.849 min was collected for HYNIC-TOC and 20.765 min for TOC were collected, lyophilised and stored at −20 °C freezer. Next the purified product was characterised by MALDI-MS, analytical HPLC, 1H NMR, 13C NMR, and FT-IR.
Radiolabelling of HYNIC-TOC
Radiolabelling [5] of peptide HYNIC conjugate with 99mTc was performed using a reducing agent SnCl2·2H2O (25 μg) and co-ligands ethylendiamine N,N diaceticacid (EDDA)/(10 mg), Tricine/(20 mg), required to stabilize 99mTc bound to the hydrazino residue of the peptide conjugate (20 μg). Labelling of HYNIC-TOC with 99mTc was performed by using various activities of 99mTc (37 MBq-370 MBq) and incubated for 15–20 min at 100 °C. The radiochemical purity assessment and quality control was achieved by RP-HPLC and using silica gel plates (ITLC-SG). HPLC technique was used to validate ITLC approach. The radiochemical purity of 99mTc-HYNIC-TOC was determined by HPLC (Waters) with reversed phase C18 column (4.6 mm × 250 mm, μ bondapak column from Waters). The mobile phase used in RP-HPLC for gradient system consists of 0.1% trifluoroacetic acid (TFA)/Water (solvent A), 0.85% trifluoroacetic acid (TFA)/acetonitrile (solvent B) at a 1 mL/min flow rate. Gradient II: 0 min 100% A (0% B), 2 min 90% A (10% B), 6 min 80% A (20% B), 12 min 50% A (50% B), 25 min 10% A (90% B), 35 min 90% A (10% B), 45 min 100%A (0% B). The solvents used for ITLC were 2-Butanone, 0.1 M sodium citrate pH 5.1 and methanol/1 M ammonium acetate (1:1) separately. Strips were analyzed in a well type gamma counter.
Stability studies
In vitro stability of the 99mTc-HYNIC-TOC complex was determined in phosphate buffer saline (pH 7.4), serum and aqueous solutions at different time points, at room temperature [6, 11, 12]. A volume of 100 μL of 99mTc-HYNIC-TOC complex solution was added to 1 mL of phosphate buffer saline (PBS) or serum and then 10 μL of this mixture was taken at each time point, added to a strip ITLC and the percentage of 99mTc-HYNIC-TOC was determined. Also the stability was validated by RP-HPLC using gradient II at different time intervals.
Determination of the partition coefficient (P) of the complex
The octanol/buffer partition coefficient was measured using a standard protocol [6]. 2 mL of 1-octanol and 2 mL PBS (pH 7.4) were mixed in a centrifuge tube. 100 μL of 99mTc-HYNIC-TOC was added to this system and vortexed at room temperature for 2 min. The mixture was incubated at 37 °C for 5 min, and then centrifuged at 5,000 rpm for 5 min. The two layers were separated; sample aliquot of 0.2 mL were taken from each phase and measured for radioactivity. The partition coefficient was calculated by dividing the radioactivity of the octanol layer with that of the water layer and log P was calculated. Care was taken to avoid cross contamination between the two phases.
Blood clearance and plasma protein binding study
Blood clearance and plasma protein binding [6, 13] of 99mTc-HYNIC-TOC was studied in rats (Sprague-Dawley) injecting 37 MBq of the radiopharmaceutical through femoral vein. The radioactivity of the withdrawn blood at different time intervals (5, 15, 30, 60, 90 and 120 min) was measured in Gamma Ray Spectrometer to obtain the blood clearance curve of the radiopharmaceuticals.
The protein binding of 99mTc-HYNIC-TOC was analysed by using centrifree micropartition devices (Amicon, Milipore) and compared with total plasma protein binding of the 99mTc-peptides by 10% trichloroacetic acid (TCA) method in Sprague-Dawley rats to determine plasma protein binding from heparinised blood. Blood cells and plasma were separated by precipitation at 10000 rpm for 6 min. Plasma proteins were precipitated and isolated by centrifugation for 6 min at 10000 rpm following the addition of an equal volume of 10% TCA in the plasma. The supernatant was decanted and the pellet was re-suspended in 1 mL TCA (10% v/v), centrifuged and the supernatant was decanted. Radioactivity in both the supernatant and pellet was measured. This count was expressed as percentage of count obtained with the same volume of unprocessed plasma.
Biodistribution study
All the experiments were conducted in accordance with the Departmental Committee of Animal Ethics and with the Institutional Guidelines of Indian Institute of Chemical Biology, Kolkata, India. Biodistribution studies were performed in rats [Sprague-Dawley rats (N = 10) 200–250 gm]. 99mTc-HYNIC-TOC complex solution (500 μCi) was administered through the femoral vein of anaesthetized rats with 0.5 mm polyethylene (PE) catheter. At different preset time intervals (2, 15, 60 and 120 min) the animals were sacrificed by intravenous injection of air. Blood and urine samples were obtained by puncturing the heart and urinary bladder, respectively. Other organs (brain, heart, liver, lungs, spleen, muscle, kidney, intestines, stomach, and pancreas) were removed, rinsed with saline and blotted dry to remove the residual blood. After that organs were weighed on a clinical scale and radioactivity was counted in a well-type counter (Gamma ray spectrometer: GRS 23C, ECIL). Standard counts of the radio-complex were also taken and the uptake was expressed in percentage of radioactivity per gram of each organ (% ID/g). The calculations of mean and standard deviation were performed on Microsoft Excel. Student’s t test was used to determine statistical significance. Differences at the 95% confidence level (p < 0.050) were considered significant.
Results and discussion
Solid phase synthesis and radiochemistry
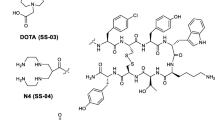
TOC (Fig. 1) and HYNIC-TOC were synthesized by FMOC strategy with an overall yield of nearly 42% and 44% respectively, based on the removal of the protecting groups after cleavage, purification and lyophilisation. HPLC chromatogram, 1H NMR, 13C NMR, IR and MALDI-MS spectra verified the composition and structural identity of purified HYNIC-peptide. After HPLC purification the purity of TOC was greater than 98% and MALDI mass was found to be m/z (M + H)+ = 1035 (Fig. 2). Also the purity of HYNIC-TOC was greater than 98% as confirmed by HPLC method and MALDI mass was found to contain m/z (M + H)+ = 1170 (Fig. 3). The HPLC elution time was 21.849 min for HYNIC-TOC (Fig. 4), 20.765 min for TOC (Fig. 5). In standard TOC (Fig. 1, procured from Commercial source), elution time was also 20.765 min (Fig. 6) and MALDI mass was also found to be m/z (M + H)+ = 1035 (Fig. 7). 1H NMR (600 MHz, D2O) δ: 8.19 (s, 1H), 7.98 (d, J = 9.6, 1H), 7.51 (d, J = 7.8, 1H), 7.47 (d, J = 8.4, 1H), 7.37 (m, 2H), 7.29–7.33 (m, 3H), 7.24 (t, J = 7.8, 1H), 7.18 (t, J = 7.8, 1H), 7.11 (s, 1H), 7.05 (d, J = 8.4, 2H), 6.92 (d, J = 9.6, 1H), 6.81 (d, J = 8.4, 2H), 4.76(1H, merged signal over lapped), 4.70 (t, J = 5.4, 1H), 4.59 (t, J = 7.8, 1H), 4.32–4.36 (m, 1H), 4.28 (d, J = 4.8, 1H), 4.25 (m, 1H), 3.98–3.99 (m, 1H), 3.88 (dd like, J 1 = 10.2, J 2 = 3.6, 1H), 3.83–3.86 (m, 1H), 3.71 (dd like, J 1 = 11.4, J 2 = 5.4, 1H), 3.62 (dd like, J 1 = 11.4, J 2 = 7.8, 1H), 3.17–3.21(m, 3H), 2.94–2.98 (m, 1H), 2.88–2.93(m, 3H), 2.84 (dd, J 1 = 13.2, J 2 = 8.4, 1H), 2.63–2.73 (m, 3H), 1.54–1.61 (m, 1H), 1.25–1.33 (m, 3H), 1.22 (d, J = 6.6, 3H), 1.14 (d, J = 6.6, 3H), 0.36 (m, br, 1H), 0.54 (m, br, 1H) ppm. 13C NMR (600 MHz, D2O) δ: 18.85 (CH3), 18.99 (CH3), 21.42 (CH2), 25.96 (CH2), 26.08 (CH2), 29.39 (CH2), 37.11 (CH2), 37.29 (CH2), 38.81 (CH2), 39.16 (CH2), 41.16 (CH2), 61.07 (CH2), 52.22 (CH), 52.57 (CH), 54.17 (CH), 54.99 (CH), 55.78 (CH), 55.81 (CH), 56.44 (CH), 59.98 (CH), 66.01 (CH), 66.48 (CH), 111.10 (CH), 115.25(CH), 118.18 (CH), 118.81 (CH), 119.32 (CH), 121.88 (CH), 124.28 (CH), 127.31 (CH), 128.82 (CH), 129.19 (CH), 130.62 (CH), 137.2 (CH), 139.92 (CH), 61.08 (C), 108.09 (C), 111.83 (C), 115.31(C), 117.25 (C), 126.56 (C), 127.87 (C), 130.64 (C), 136.04 (C), 136.12 (C), 139.93 (C), 154.50 (C), 155.41 (C), 162.86 (C), 163.10 (C), 165.65(C), 169.97 (C), 171.95 (C), 172.13 (C), 172.26 (C), 172.57 (C), 174.61 (C), 174.98 (C) ppm. IR spectra (KBr): 3287, 1664, 1536, 1200 cm−1. The IR, 1H NMR, 13C NMR and mass spectral data of HYNIC-TOC were supported the structure as given in (Fig. 8).
The radiolabelling efficiency i.e. the percentage of the 99mTc-HYNIC-TOC in the preparation was more than 98%, when determined by ITLC. ITLC chromatograms did not show any changes in radiochemical purity during the time course considered for the analysis and the values were higher than 98%. We obtained high radiochemical yield (98.49 ± 0.2%) with very low amount of 99mTc-pertechnetate (0.1 ± 0.02%), 99mTc-radiocolloid (0.5 ± 0.08%) and 99mTc-coligand (1.0 ± 0.02%). In RP-HPLC analysis of 99mTc-HYNIC-TOC (Fig. 9) we observed a single major peak at 13.138 min (elution time) with minor impurities, which was stable up to 24 h post-labelling period in the room temperature. In ITLC 2-Butanone solvent, the labelled 99mTc-HYNIC-TOC, 99mTc-coligands and 99mTc-colloid remains at the origin (R f = 0) and 99mTcO4 − moves at the solvent front (R f = 1), for 0.1 M sodium citrate, pH 5.1 solvent system, the labelled 99mTc-HYNIC-TOC and 99mTc-colloid remains at origin (R f = 0) and 99mTc-coligands and 99mTcO4 − moves at the solvent front (R f = 1) and for methanol/1 M ammonium acetate (1:1) solvent system, 99mTc-colloid remains at the origin (R f = 0) and labelled 99mTc-HYNIC-TOC, 99mTc-coligands and 99mTcO4 − moves at the solvent front (R f = 1) (Table 1).
From the stability study we found 99mTc-HYNIC-TOC complex was stable in phosphate buffer saline (pH = 7.4), serum and aqueous solutions for 24 h post labelling at room temperature [10].
Partition coefficient (P) of the complex, protein binding and renal clearance study
Log P, which is a measure of lipophilicity, was calculated from the Octanol/water partition coefficient method partition coefficient of the 99mTc-HYNIC-TOC complex was −1.27 ± 0.12 at pH 7.4 and has showed no significant difference with in the pH range 7.0–7.4.
The extent of free 99mTc-HYNIC-TOC in rat plasma was measured by trichloro acetic acid (TCA) and also by Amicon Ultrafiltration unit. TCA method provided the total bound protein while the other gave the plasma binding level. The percentage of protein bound drug was found to be 56% by TCA method and 54% by Amicon Ultrafiltration method in the blood plasma of Sprague-Dawley rat. The blood clearance curve of 99mTc-HYNIC-TOC complex showed rapid clearance from the blood at 25 min and then it gradually decreases showed in (Fig. 10).
Biodistribution in rat
Biodistribution results of 99mTc-HYNIC-TOC were obtained in Spargue Dawley rats administered intravenously and sacrificed at 2, 15, 60, 120 min post administration as the percentage of the Injected Dose/Gram of organ (% ID/g). Results showed higher uptake in kidney than any other organs. The Kidney uptake (% ID/g) was 4.58 ± 0.14, 4.51 ± 0.16, 4.45 ± 0.03 and 2.99 ± 0.01 at 2, 15, 60, 120 min post administration. The blood clearance was faster with rapid excretion through kidneys and relatively low uptake in liver. Quite low uptake was observed in stomach (0.110 ± 0.56, 0.140 ± 0.04, 0.038 ± 0.01, 0.021 ± 0.03) and intestine (0.135 ± 0.06, 0.15 ± 0.10, 0.02 ± 0.06) at all the time points, indicating a minimal in vivo decomposition of the chelate to form free 99mTcO4 −/99mTcO2 (Fig. 11).
Conclusions
Thus, we have successfully synthesised highly pure HYNIC-TOC and TOC. We have also prepared 99mTc-HYNIC-TOC complex with high radiochemical purity and yield. The synthesis and cyclisation of HYNIC-TOC and TOC were done successfully by indigenous SPPS method. 99mTc-HYNIC-TOC was radio-chemically and biologically evaluated. So HYNIC-TOC can be utilized for preparation of cost effective, stable and single vial dry kit formulation that can be easily labelled with 99mTc for clinical use.
References
Behr TM, Behe M, Becker W (1999) Q J Nucl Med 43:268–280
Arano Y, Akizawa H (1997) Bioconju Chem 8:442–446
Chandra S, De K, Ganguly S, Sarkar B, Misra M (2009) Peptides 30(12):2399–2408
Abrams MJ, Juweid M, TenKate CI, Schwartz DA, Hauser MM, Gaul FE, Fuccello AJ, Rubin RH, Strauss HW, Fischman AJ (1990) J Nucl Med 31:2022–2028
Gandomkar M, Najafi R, Babaei MH, Shafiei M, Sadat Ebrahimi SE (2006) DARU 14(4):183–189
De K, Chandra S, Sarkar B, Ganguly S, Misra M (2010) J Radioanal Nucl Chem 283:621–628
Storch D, Behe M, Walter MA, Chen J, Powell P, Mikilajczak R, Macke HR (2005) J Nucl Med 46:1561–1569
Schottelius M, Schwaiger M, Wester HJ (2003) Tetrahedron Lett 44:2393–2396
Lee TW, Chang SR, Chen ST, Tsai ZT (1998) Appl Radiat Isot 49(12):1581–1586
Decristoforo C, Mather SJ (1999) Biconjugate Chem 10:431–438
Hunt AP, Frier M, Johnson RA, Brezenko S, Perkins AC (2006) Eur J Pharm Biopharm 50:227–236
Teran M, Savio E, Paolino A, Frier M (2004) Eur J Pharm Biopharm 57:347–352
Ertay T, Unak P, Tasci C, Zihnioglu F, Durak H (2005) Appl Radiat Isot 62:883–888
Acknowledgments
The authors gratefully acknowledge Prof. Siddhartha Roy Director, Indian Institute of Chemical Biology (IICB), Kolkata, India and Dr. N. Sivaprasad, Sr. General Manager, Board of Radiation and Isotope Technology (BRIT), Navi Mumbai, India for their support. Also thanks to Mr Indranil Banerjee, junior research fellow of Indian Institute of Chemical Biology (IICB), Kolkata, India for technical support. We also thank Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
M. Misra—first corresponding author.
Rights and permissions
About this article
Cite this article
Behera, A., De, K., Chandra, S. et al. Synthesis, radiolabelling and biodistribution of HYNIC-Tyr3 octreotide: a somatostatin receptor positive tumour imaging agent. J Radioanal Nucl Chem 290, 123–129 (2011). https://doi.org/10.1007/s10967-011-1156-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1156-1