Abstract
The over-expression of folate receptors in variety of neoplastic tissues makes radiolabeled folate conjugates potential agents for imaging and therapy of such cancers. With the aim of preparing an imaging agent for targeting folate receptors, folic acid has been conjugated with homocysteine for complexation with [99mTc(CO)3(H2O)3]+ core. The radiolabeled complex of the homocysteine-folate could be obtained in >95% radiochemical yield as observed by HPLC. Stability of complex in saline was studied and challenge studies with histidine and cysteine revealed kinetic stability of the complex. Lipophilicity of the radiolabeled complex (log P) was found to be 0.45. In vitro uptake of 99mTc(CO)3-labeled folic acid derivative was studied in KB cells and inhibition studies were carried out using 3H-folic acid and cold homocysteine–folate conjugate. The in vitro studies indicated loss of binding affinity of the derivative towards folate receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin B9, folic acid is required for DNA synthesis and plays an important role in cell proliferation [1]. Hence, in highly proliferating tumor cells, folate-binding protein (FBP) that acts as a receptor for folic acid is upregulated [2, 3]. These high affinity folate receptors (FR) are over-expressed in various human cancers including breast, ovarian, cervical, colorectal, renal, and nasopharyngeal tumors with very limited expression in normal tissues [4–6]. The FR density also appears to increase as the stage/grade of the cancer increases. Folate derivatives bind to folate receptors with high affinity and enter into cells by receptor-mediated endocytosis pathway [7–9]. Folate receptor is thus a potential molecular target for preparing tumor targeting radiolabeled folic acid conjugates. Such radiolabeled conjugates can be excellent diagnostic agents for imaging of receptor-positive tumor cells as well as monitoring of tumor response to the treatment.
The folate receptor has been targeted using radiolabeled tumor-specific monoclonal antibodies, (MOv18, MOv19) which have been evaluated clinically [10]. However, radiolabeled low molecular weight folic acid conjugates targeting folate receptor positive tumors offer advantages of having small size, being non-immunogenic and exhibiting faster systemic blood clearance kinetics as compared to that of monoclonal antibodies. This, in turn leads to increased tumor to background ratios. Several folate tracers radiolabeled with 66/67/68Ga, 111In, 64Cu, and 99mTc are under pre-clinical and clinical stages of development for imaging of tumors which are positive for folate receptors [11–13]. 111In-labeled diethylenetriamine pentaacetate-(DTPA)-folate is the most extensively studied radiolabeled folate conjugate and is under phase II clinical trials [14]. Although 111In-(DTPA)-folate has shown encouraging results, 99mTc is a more suitable radionuclide for imaging purposes due to its ideal physical decay characteristics (E γ = 140 keV, t 1/2 = 6 h) as compared to that of 111In (E γ = 245 keV, t 1/2 = 2.8 days). Additionally the easy availability of 99mTc from a 99Mo-99mTc generator system at low cost is an added advantage for developing 99mTc-based radiopharmaceuticals. Another advantage of 99mTc is its chemical similarity with its congener, 188Re (t 1/2 = 17 h, E β = 2.12 MeV, E γ = 155 keV) which is a particulate emitter and hence can be used as a therapeutic radioisotope. Amongst the various chemical forms and cores of technetium used for radiolabeling, [99mTc(CO)3(H2O)3]+ has gained interest because of the presence of low spin d6 Tc(I) center that leads to formation of kinetically inert complexes in vivo [15, 16].
In the present work, we describe the derivatization of folic acid to introduce a tridentate chelating agent for radiolabeling with 99mTc(I) tricarbonyl precursor and in vitro evaluation of the resulting radiolabeled complex.
Experimental
All reagents used were of analytical reagent (AR) grade. Folic acid was obtained from Sigma–Aldrich (USA). Carbon monoxide in 0.5 L refillable canisters was obtained from M/s Alchemie Gases & Chemicals (Mumbai, India). 99mTcO4 − was eluted from an in-house 99Mo/99mTc column generator using normal saline. High Performance Liquid Chromatography (HPLC) analyses were performed on a Jasco PU 1580 system and a Jasco 1575 tunable absorption detector and a radiometric detector system having C-18 reversed phase HiQ Sil (5 μ, 250 × 4 mm) column. The gradient system consisting of eluting solvents triethylammonium phosphate buffer (TEAP: 0.05 M, pH7) (solvent A) and methanol (solvent B) was used for HPLC analyses (0 min: 100% A; 0–30 min: 100% A-20% A). Proton NMR spectra were recorded on 300 MHz Varian VXR 300S spectrophotometer. KB (Human nasopharyngeal carcinoma) & A375 (Human lung carcinoma) cell lines were procured from National Centre for Cell Sciences (NCCS), Pune, India. 3H-Folic acid (0.74 MBq/ml) was supplied by Board of Radiation in Isotope Technology (BRIT), Navi Mumbai, India. Cell culture reagents were from Sigma and tissue culture flasks/accesories were from Nunc. 99mTc activity was counted in NaI(Tl) Scintillation counter while Liquid Scintillation counter from Hewlett Packard was used for measuring 3H activity.
Synthesis of homocysteine-folate conjugte
To a solution of folic acid (220 mg, 0.49 mmol) in dimethylsulfoxide (DMSO) (5 mL), N-hydroxysuccinimide (57 mg, 0.49 mmol) and Dicyclohexylcarbodiimide (DCC) (206 mg, 1 mmol) were added. The reaction flask was wrapped in aluminium foil and the mixture stirred at room temperature for 6 h. Subsequently the precipitate of dicyclohexylurea was filtered and homocysteine (66 mg, 0.49 mmol) dissolved in DMSO was added to it in three portions. The reaction mixture was stirred at room temperature for 24 h followed by addition of acetonitrile which resulted in formation of greenish yellow precipitate that was filtered and washed with diethyl ether. 1H-NMR (DMSO, δ ppm): 8.65 (s, 1H), 7.7 (t, 2H), 6.6 (t, 2H), 4.55 (s, 2H), 4.5 (t, 1H), 3.5 (t, 1H), 2.3 (t, 2H), 2.1 (t, 2H), 1.24 (m, 2H), 1.1 (m, 2H). ESI MS m/z: 581 [M + Na]+.
Preparation of [99mTc(CO)3(H2O)3]+ precursor
The radioprecursor synthon was prepared by a modified procedure reported by Alberto et al.16 An aqueous solution (0.5 mL) of NaBH4 (5.5 mg), Na2CO3 (4 mg) and Na/K tartrate (15 mg) was purged with carbon monoxide gas for 5 min, followed by addition of 1 mL of 99mTcO4 − (~37 MBq, 1 mCi). The reaction mixture was heated at 80 °C for 20 min. The pH of the reaction mixture was adjusted to 8 with 300 μL of 0.5 M phosphate buffer (pH 7.5): 1 M HCl (1:3 v/v).
Preparation of 99mTc(CO)3-homocysteine folate complex
To 0.1 mL methanolic solution of folic acid conjugate (200 μg, 0.25 μmol), 0.4 mL of [99mTc(CO)3(H2O)3]+ was added and the pH of the resultant mixture was adjusted to 6 followed by heating at 70 °C for 20 min. The complex prepared was characterized by HPLC.
Lipophilicity studies
The lipophilicity of the complex was studied using solvent extraction where 0.2 mL of the complex was taken in 0.8 mL saline and 1 mL octanol was added to it. The mixture was vortexed and equal aliquots (100 μL) of octanol and saline were withdrawn and counted to measure the radioactivity in each layer. The organic extract was back extracted in saline repeatedly to estimate the distribution ratio.
Stability studies
The 99mTc(CO)3-homocysteine folate complex, was incubated at 37 °C for 6 h. The stability of the complex was studied by monitoring the elution profile in HPLC.
Histidine and cysteine challenge studies
In order to study the stability of 99mTc(CO)3-homocysteine folate complex towards exchange with other challenging ligands such as histidine and cysteine, solutions (10 μL, 0.5 M) in distilled water were added to 100 μL of complex and the resultant mixtures were incubated at 37 °C for 1 h. The mixtures were analyzed by HPLC in order to estimate the extent of exchange, if any.
In vitro studies
In vitro studies were carried out in KB cells that over-express folate receptors while A375 cells were used as control. KB cells were cultured in Rosewell Park Memorial Institute (RPMI) medium without folic acid, supplemented with 10% Fetal Calf Serum FCS). Cells were grown to confluence in a humidified atmosphere with 5% CO2 at 37 °C. Trypsin–EDTA solution was used for sub-culturing and isolation of cells. 20 h prior to the experiment, ~8 × 105 cells/well were seeded in 12 well plates and incubated at 37 °C to form confluent monolayer overnight. Cell monolayers were rinsed with ice cold PBS (pH 7.4) and were fed with pure RPMI medium without FCS. 99mTc(CO)3-folic acid conjugate (25 μL, 1 MBq/mL) was added to the cells and plates were incubated at 37 °C for 1 h. Blocking experiments were carried out by pre-incubating cells with excess of folic acid (100 μM) for 30 min in order to saturate FRs. Cell monolayers were washed with PBS for determining the total bound activity and with stripping buffer (aqueous solution of 0.1 M acetic acid & 0.15 M NaCl pH 3) to determine the internalized activity. Monolayers were then dissolved in 1 N NaOH. Radioactivity associated with the cells and the supernatant were counted in NaI(Tl) scintillation counter.
The inhibition studies were carried out using five different concentrations of cold folic acid and folic acid derivative (0, 0.002, 0.01, 0.1, 0.4 mM) which were added to the cells in well plates. Cells were incubated at 37 °C for 40 min. 3H-Folic acid (25 μL, 0.8 μM/7.4 KBq) was added to each well and plates incubated at 37 °C for 1 h. After incubation, cells were rinsed with ice cold phosphate buffer saline (PBS) and the monolayer was dissolved in DMSO. Scintillation cocktail was added after transferring the cells in vials and 3H activity was counted in Liquid Scintillation counter.
Results and discussion
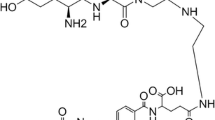
The expression of high levels of folate receptors in ovarian carcinoma and other epithelial carcinomas makes it a potential target for development of radiolabeled folate conjugates for use in diagnostic imaging as well as targeted therapy. Since folic acid does not have suitable functional groups for radiolabeling with organometallic [99mTc(CO)3(H2O)3]+ precursor it was derivatized to introduce homocysteine as a tridentate chelating ligand for complex formation (Scheme 1). The functionalization of folic acid was carried out by initial activation of carboxyl group using N-hydroxysuccinimide. The N-hydroxysuccinimidyl ester formed was characterized by 1H-NMR where succinimidyl protons appeared at 2.83δ. The activated ester was then conjugated with homocysteine, leading to formation of the corresponding amide derivative. The 1H-NMR of amide derivative showed appearance of a triplet at 2.3δ and a multiplet at 1.24δ corresponding to protons of two methylene groups of homocysteine. Thus, the formation of the folic acid–homocysteine conjugate was confirmed. This conjugate offers a tridentate array of N, S, O donor atoms for coordination with [99mTc(CO)3(H2O)3]+ core.
Taking advantage of the stability of the 99mTc-tricarbonyl core and complexes formed thereof, [99mTc(CO)3(H2O)3]+ has been selected as a precursor of choice for radiolabeling of folic acid derivative. The technetium-tricarbonyl core was prepared according to the reported procedure (Alberto et al., 1998) by purging carbon monoxide gas. It could be prepared in >98% yield as determined by C-18 reversed phase HPLC system. The retention time of 99mTcO4 − and [99mTc(CO)3(H2O)3]+ precursor in TEAP buffer and methanol as eluting solvents were 3.5 min and 4 min respectively (Fig. 1a). The 99mTc(CO)3-homocysteine folate complex was prepared by incubation of folic acid derivative with the [99mTc(CO)3(H2O)3]+ synthon at pH 6. The radiolabeled complex was eluted out as single species in HPLC with retention time of 14 min, in >95% yield (Fig. 1b). Lipophilicity of the radiolabeled complex determined by distribution in octanol and water and the partition coefficient (log P) was found to be 0.45. In vitro stability of the complex was studied in phosphate buffer saline at pH 7.0 up to 24 h wherein the retention of RC-purity of the complex could be observed from the HPLC pattern. The histidine and cysteine challenge studies were carried out to determine the extent of in vivo decomposition of the complex if any, in presence of excess of these amino acids. The incubation of the complex with these ligands did not result in any change in the HPLC pattern and also there was no indication of formation of a second species in form of 99mTc(CO)3-histidine and 99mTc(CO)3-cysteine complex in HPLC. Thus, the studies indicate the kinetic inertness of the complex in presence of challenging ligands as could be present under in vivo conditions.
99mTc(CO)3-homocysteine folate complex was tested for its uptake in KB cell line which is widely reported for evaluating folate analogues. The percentage of the radiolabeled complex associated with cells and results of corresponding inhibition are tabulated in Table 1. Uptake of 3.93 ± 0.43% and corresponding inhibition of ~49% was observed in binding studies with KB cells. However, the uptake of 99mTc(CO)3-homocysteine folate complex in KB cells was less compared to that in A375 cell line which does not express any folate receptors indicating nonspecific binding of the complex with KB cells. Results of inhibition studies with 3H-Folic acid and unlabeled homocysteine-folate conjugate are depicted in Fig. 2. A sharp decrease in the uptake of 3H-Folic acid in KB cells was observed on increasing the concentration of folic acid as expected due to preoccupancy of the receptors. However, no decrease in the cell uptake of 3H-Folic acid was observed on increasing the concentration of folic acid-homocysteine conjugate, indicating a possible loss of binding affinity of the folate-homocysteine conjugate towards folate receptors.
Conclusion
Folic acid has been derivatized to incorporate an array of donor atoms constituting a tridentate chelator for formation of a stable complex with [99mTc(CO)3(H2O)3]+ core. The radiolabeled complex obtained in >95% radiochemical yield has been evaluated towards determining its affinity for folate receptors in in vitro systems. Inhibition studies revealed loss of binding affinity of the radiolabeled folic acid derivative.
References
Leamon C, Low PS (2001) Drug Discov Today 6:44–51
Leamon CP, Low PS (1991) Proc Natl Acad Sci USA 88:5572–5576
Leamon CP, Low PS (1993) Biochem J 291:855–860
Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR Jr, Kamen BA (1992) Cancer Res 52:3396–3401
Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M (1997) Int J Cancer 74:193–198
Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP (2005) Anal Biochem 338:284–293
Rothberg KG, Ying YS, Kolhouse JF, Kamen BA, Anderson RG (1990) J Cell Biol 110:637–649
Antony AC (1992) Blood 79:2807–2820
Jansen G (1999) In: Jackman AL (ed) Antifolate drugs in cancer therapy. Humana Press, Totowa, NJ, pp 293–321
Crippa F, Buraggi GL, Dire E, Gasparini M, Seregni E, Canevari S, Gadina M, Presti M, Marini A, Seccamani E (1991) Eur J Cancer 27:724–729
Guo W, Hinkle GH, Lee RJ (1999) J Nucl Med 40:1563–1569
Wang S, Lee RJ, Mathias CJ, Green MA, Low PS (1996) Bioconjug Chem 7:56–62
Wang S, Luo J, Lantrip DA, Waters DJ, Mathias CJ, Green MA, Fuchs PL, Low PS (1997) Bioconjug Chem 8:673–679
Siegel BA, Dehdashti F, Mutch DG, Podoloff DA, Wendt R, Sutton GP, Burt RW, Ellis PR, Mathias CJ, Green MA, Gershenson DM (2003) J Nucl Med 44:700–707
Alberto R, Schibli R, Egli A, Schubiger PA (1998) J Am Chem Soc 120:7987–7988
Alberto R, Schibli R, Waibel R, Abram U, Schubiger PA (1999) Coord Chem Rev 190–192:901–919
Acknowledgments
The authors express their sincere thanks to Dr. V. Venugopal, Director, Radiochemistry & Isotope Group, BARC for his support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satpati, D., Mukherjee, A., Venkatesh, M. et al. Radiosynthesis and in vitro evaluation of 99mTc(CO)3-labeled folic acid derivative. J Radioanal Nucl Chem 290, 89–93 (2011). https://doi.org/10.1007/s10967-011-1142-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1142-7