Abstract
Radiochemical separation of Pu, Am and U was tested from synthetic solutions and evaporator concentrate samples from nuclear power plants for isolation of each of them for alpha-spectrometry analysis. The separation was performed by anion-exchange chromatography, extraction chromatography, using TRU resin, and precipitation techniques. The aim of the study was to develop a sensitive analytical procedure for the sequential determination of 242Pu, 238Pu, 239+240Pu, 241Am and 235, 238U in radioactive wastes. 238Pu, 242Pu, 243Am and 232U were used as tracers. The measurements of α emitting radionuclides were performed by semiconductor detector that is used especially when spectrometric information is needed. For synthetic solutions the chemical recovery was based on associated iron concentration and was about 93%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Final disposal of low and intermediate level (LLW and ILW) radioactive waste have to meet certain requirements of regulations and one of this is the specification of radionuclide inventory of nuclear power plant. The safety planning for disposal takes account in special long half life radionuclides. For the control of them in waste packages is necessary to insure the respect of waste acceptance criteria that are fixed in order to avoid any potential impact of the radio contaminants on the environment of the repository site.
Many of the important long lived radionuclides contained in the radioactive waste are difficult to measure (DTM) from outside the waste packages using non-intrusive techniques because they are low energy, non-penetrating beta or alpha emitting nuclides (i.e. non-gamma emitters). Identification of these DTM nuclides requires methods that, in general, involve analysis of waste samples using complex chemical analysis to separate the various radionuclides for measurement.
The aim of this study was to develop a rapid and sensitive analytical procedure for simultaneous determination of Pu, Am and U radioisotopes in waste samples from nuclear power plants based on alpha spectrometry following radiochemical separation by ion exchange and extraction chromatography.
Simultaneous determinations, where several elements can be determined simultaneously in the same sample preventing that calcinations and separation procedure occur for each element, are the most desirable methods because they are not time consuming and they are low cost. There are several methods available for the determination of isotopes of Pu, Am and U in environmental and bioassay samples [1–3]. However few of them have considered nuclear wastes samples besides iron as potential interference in radiochemical separations [1, 4, 5].
Alpha-spectrometry is normally used for the determination of 238Pu and 239+240Pu. Due to the interferences, the radionuclide of interest must be isolated of the matrix and other interfering radionuclides before measurement. The separation of Pu by ion exchange chromatography is based on the formation of anionic complexes of Pu(IV) with NO3 – an Cl– in concentrated HNO3 or HCl. Alpha-spectrometry is a more sensitive method for determination of 241Am. However, it must be separated from matrix and interfering radionuclides. The separation can be made by co-precipitation of hydroxides or oxalate, solvent extraction using TIOA(triisooctylamine)/xylene, anion exchange and extraction chromatography. Also, for uranium isotopes alpha-spectrometry has been used for a long time, being the technique well established and very reliable as it presents only a few problems. Therefore each of the radionuclides has their peculiar behavior that allows isolation and sequential isotopic determination.
Standards solutions of Pu, Am, U, Sr, Fe and sample of evaporator concentrate were analyzed to study the separation factors and interferences in the measurement of Pu, Am and U isotopes.
Experimental
Reagents and apparatus
All reagents used were analytical grade. Certified radionuclides were obtained from the Instituto de Radioproteção e Dosimetria (IRD), Rio de Janeiro, Brasil and the certified activity level for each of them is: 238Pu (0.56 kBq), 242Pu (0.02 kBq), 243Am (4.20 kBq), 232U (4.26 kBq), 90Sr (2.49 kBq) and 55Fe (2.84 kBq). Working solutions were prepared by transferring a known weight of the tracer followed by volumetric dilution to an appropriate working concentration.
The alpha-spectrometry measurements were carried out with Canberra PIPS (passivated ion implanted planar silicon) detectors. The alpha-energy calibration and the measurement of counting efficiency of the detector were performed using a source from Analytics Inc. Analytics maintains traceability to the National Institute of Standards and Technology through Measurements Assurance Programs as described in USNRC Regulatory Guide 4.15, Rev. 1 [6]. The spectrometer used was a Canberra Model S509 Genie-2000 Alpha Analyst.
Sample Preparation
Synthetic solutions were prepared from working solutions of 238Pu, 243Am, 232U and 55Fe radionuclides and Fe (3 mg/L) as yield monitor. Mixture of 40 g of evaporator concentrate (EC) and 238Pu or 242Pu, 232U and 243Am as tracers were prepared in a Pt crucible and heated using a hot plate up to evaporation to dryness. After that, 20 mL of nitric acid and 15 mL of H2O2 were added into the crucible. The mixture was heated again up to evaporation to dryness. The solid obtained was calcined in an oven at 850 °C for 4 h. The residue was dissolved and for each step after addition of acid the mixture was heated up to evaporation to dryness: first, with 30 mL of 3:2 nitric acid, after, with 15 mL of HF. Finally, the solid resultant was dissolved using a hot plate in 100 mL of 3:2 nitric acid.
Radionuclides isolation
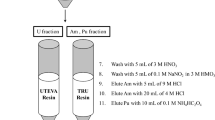
The radiochemical procedure [2] consists of a Pu isolation step by an anion exchange column (Dowex 1X8, Cl-form, 100–200 mesh, Sigma Chemical Co., USA). The solution, 100 mL in 3:2 nitric acid, is passed through the column and rinsed with 120 mL of 3:2 nitric acid. The effluent is retained for strontium, iron, americium and uranium isolation. Pu was removed from the column by reduction with a freshly prepared mixture of ammonium iodide [5%(w/v)] and concentrated hydrochloric acid (29:71 by volume). Iodide ions selectively reduce Pu(IV) to Pu(III), which is not adsorbed on the column under these conditions and elutes.
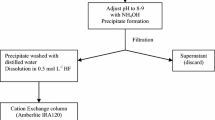
The effluent from the anion exchange column was used to separate Am and Sr by co-precipitation with oxalic acid when the pH of the solution was adjusted to 5.5–6.0 with 25% ammonia solution [3]. Iron is an interfering element for americium isolation and under these conditions in many cases, most of the Fe remains in solution in the form of oxalate complexes. After filtration, the precipitate was used for americium isolation by using a TRU resin extraction chromatography column (Eichrom Industries Inc. USA). If iron is present before elution of the solution ascorbic acid is added to reduce Fe(III) to Fe(II).
The filtrate retaining uranium and iron fractions was heated to dryness and the solid was dissolved in 30 mL of concentrate nitric acid and heated to dryness to destroy the excess of oxalic acid. The solid obtained was hot dissolved in 30 mL of 3:2 nitric acid and was diluted to 200 mL with deionized water. The pH of the solution was corrected to 9.0 with ammonia hydroxide for co-precipitation of iron hydroxide and uranium. The precipitate was filtered and the filtrate was dissolved in 20 mL 9 M HCl. They were separated by anion exchange column (Dowex 1X8, Cl-form, 100–200 mesh, Sigma Chemical Co., USA) that was loaded with the solution and either was stripped iron with 50 mL of 8 M nitric acid and uranium with 100 mL of 0.1 M HCl. The flow chart of combined procedure for the determination of Pu, Am and U radionuclides is shown in the Fig. 1.
Determination of Pu, Am and U
Determination of Pu, Am and U was carried out by using alpha-spectrometry. Solutions of the isolated radionuclides were evaporated to dryness and dissolved in a mixture of nitric acid, 10 mL, and perchloric acid, 5 mL. The samples were evaporated to dryness and retaken in a solution of 3 M H2SO4 and 0.8 M (NH4)2SO4 with the pH adjusted in a range of 1.2–2.8 (Thymol blue indicator) with concentrate NH4OH. Electrodepositions disks were prepared from 0.90 mm thick sheets of stainless steel, by machining sections to 24.8 mm diameter planchets. The electroplating was done at an applied current of 1.0 A for U and Am and 1.2 A for Pu for 1 h.
The counting time was 150,000 s for all samples. The lower limit of detection (LLD) [7] was determined using the following equation modified from Currie [8] at the 95% confidence level for an alpha spectrometric analysis:
where, σ B is the standard deviation of the total background counts without sample in the chamber; T is the background counting time (min); eff is the counting geometry (cpm/dpm); Y is the assumed fractional chemical yield; and, wt is the assumed sample weight (grams). The yield and the final activity concentration was calculated according to Vesterbacka [9].
Results and discussion
Among nuclear techniques, alpha-spectrometry offers a high-energy resolution. The use of low background semiconductor detectors makes this technique particularly sensitive for the measurement of very low activities of actinides. Although the resolution of detectors for alpha-spectrometry is good, the relatively small difference in α particle energy between some α emitters makes it difficult to spectrometrically separate the peaks [10]. The simultaneous availability of new resins improves the element separation selectivity, particularly the development of sequential steps to extract and purify Pu isotopes, 241Am and U isotopes [11]. The combined high-resolution with the good chemical separation of radionuclides of interest makes the technique very attractive to be used for the measurement of α emitting radionuclides.
The method used here combines the well established procedure for Pu analysis based on anion exchange, the application of the TRU-Spec column for separation and purification of the Am fraction and the anion exchange for U isolation. In order to determine chemical recovery of Pu, the evaporator concentrate was spiked with a known activity of 242Pu or 238Pu. The chemical recoveries [7] for Pu are usually higher than 60% and show narrow dispersion. In the analysis for Am a crucial point lies in the influence of Fe(III) on the Am uptake on TRU-Spec column. For synthetic solutions, the recovery of Fe(III) were about 93%. That is, following the separation procedure part of the iron co-precipitate with calcium oxalate. In this case ascorbic acid was used to reduce Fe(III) in nitric solutions. The reduction of Fe(III) allows the maximum retention of americium on the stationary phase [3].
The experiences with synthetic solutions provided a way to compare alpha-spectra obtained for radionuclides with the aim of their isolation and characterization, due to the high resolution of the PIPS detector. For the radionuclides tested in synthetic solutions using 238Pu, 242Pu, 243Am and 232U, the separation process produces high-quality spectra in terms of good energy resolution and without interference lines. Typical spectrum obtained by alpha-spectrometry after isolation of Pu isotopes, for a sample of evaporator concentrate spiked with 242Pu, 243Am and 232U is shown in the Fig. 2.
There is a good separation of the radionuclides present in the initial sample. The resolution of alpha-spectra is 25.63 keV at 4.908 MeV for the 242Pu peak, 26.41 keV at 5.512 MeV for the 238Pu peak and 38.29 keV for the 239+240Pu peak in terms of the full width at half maximum (FWHM). These resolutions were obtained without oxidation state adjustment [12]. Besides, it is noticed that there are no peaks that can be marked to Am or U, the others α emitting radionuclides presents in the initial sample.
For one typical sample of EC, the results for a sequential analyses of 238Pu, 239+240Pu, 242Pu, 241Am, 235 and U 238U are shown in Table 1. When the real samples were analyzed for these radionuclides there is not any interference in the measurement by alpha-spectrometry. The alpha spectra from the Pu fractions have good resolution (40–50 FWHM) and show neither Am or U contamination. The disintegration rate of the analyte activities in each sample is calculated on the basis of the known tracer activities added to the sample. In general, for evaporator concentrate samples the values found for activity of the radionuclides were always larger than the LLD.
Table 2 shows activities for 239+240Pu and 238Pu and their radiometric yields as a result of analyses using the 242Pu as tracer for different samples. Although evaporator concentrate as reference material certified for them had not been used, the recoveries of Pu isotopes in the process of analyses are measured by radiometric yields of the tracers spiked in the samples. The accuracy of the determination of Pu isotopes in the samples directly depends on the uncertainty in the 242Pu tracer concentration value. Thus, the standard solution of certified radionuclides (IRD) was used to prepare diluted solutions to appropriate concentration. In spite of this, according to the values presented in Table 2 the radiometric yield varied for three replicate determinations. The great difference of the yields and the high relative standard deviation for determinations are probably coming from individual analysis method of the nuclide [2] and the natural heterogeneity of evaporator concentrate from nuclear power plants [13], respectively. Anyway, the tracer recovery combined with high-resolution ensures a good reliability of the measurement, even at very low levels.
We can compare the spectrum shown in Fig. 2 with two spectra obtained in different situations: first, the lack of the peak for the 242Pu was observed when the tracer is changed for 238Pu, Fig. 3; and, second, keeping the same activity for the tracer but changing the initial volume taken for the sample of 40 g to 100 g, Fig. 4.
For the second case the specific activities and FWHM are shown in the Table 3. That is, a large sample volume of evaporator concentrate was not required for the analysis, but significantly smaller volume was enough to determine the activities for Pu isotopes. Therefore, this method makes it possible to use smaller amounts of hazardous material with minimum waste generation.
Conclusions
In this study, Pu, Am and U were analyzed by radiochemical separation followed by alpha-spectrometry spectra with good resolution. The method of sequential analysis provided an excellent purification of each radionuclide. Therefore, further improvements of the method are foreseen as it is necessary to develop accurate and reliable methods for the determination of actinides in the low and medium radioactive wastes that arise from nuclear power plants.
References
Tavcar P, Smodik B, Benedik L (2007) Radiological characterization of low- and intermediate-level radioactive wastes. J Radioanal Nucl Chem 273(3):593–596
Wang JJ, Chen IJ, Chiu JH (2004) Sequential isotopic determination of plutonium, thorium, americium, strontium and uranium in environmental and bioassay samples. Appl Radiat Isot 61: 299–305
Moreno J, Vajda N, Danesi PR, Larosa JJ, Zeiller F, Sinojmeri M (1997) Combined procedure for the determination of 90Sr, 241Am and Pu radionuclides in soil samples. J Radioanal Nucl Chem 226(1–2):279–284
Tagami K, Uchida S (2004) Use of TEVA resin for the determination of U isotopes in water samples by Q-ICP-MS. Appl Radiat Isot 61:255–259
Singhal RK, Narayanan U, Karpe R, Kumar A, Ranade A, Ramachandran V (2009) Selective separation of iron from uranium in quantitative determination of traces of uranium by alpha spectrometry in soil/sediment sample. Appl Radiat Isot 67:501–505
Analytics, Certificate of Calibration 75551–121, 2007
International Atomic Energy Agency, International Labour Office (1999) Assessment of occupational exposure due to intakes of radionuclides. Laboratory Practices Module VI.2/2, IAEA, Vienna
Currie LA (1968) Limits for quantitative detection and quantitative determination. Anal Chem 40:586–593
Vesterbacka P, Klemola S, Salahed-Din K, Saman M (2009) Comparison of analytical methods used to determine 235U, 238U and 210Pb from sediment samples by alpha, beta and gamma spectrometry. J Radioanal Nucl Chem 281:441–448
Hou X, Roos P (2008) Critical comparison of radiometric and mass spectrometric methods for the determination of radionuclides in environmental, biological and nuclear waste samples. Anal Chim Acta 608:105–139
Goutelard F, Morello M, Calmet D (1998) Alpha-spectrometry measurement of Am and Cm at trace levels in environmental samples using extraction chromatography. J Alloy Compd 271–273:25–30
Taddei MHT, Silva NC (2006) Radiochemical procedure for the determination of plutonium isotopes in powdered milk. J Radioanal Nucl Chem 269(2):457–461
Rodríguez M, Piña G, Gallego C, Bienvenu P, Combes C, Excoffier E, Gysemans M, Vanderlinden F, Voors PI, Fachinger J, Maischak S, Dobbels F, Ervanne H, Dale C, Klein F, Ferrando M (2003) Interlaboratory radiochemical analysis comparison on a primary waste flux. Luxembourg: European Commission, (EUR-20616)
Acknowledgments
The authors are very grateful to Eletrobrás Termonuclear for its collaboration and samples supply and to Instituto de Radioproteção e Dosimetria (IRD) – CNEN for its radiotracer standards supply.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reis, A.S., Temba, E.S.C., Kastner, G.F. et al. Combined procedure using radiochemical separation of plutonium, americium and uranium radionuclides for alpha-spectrometry. J Radioanal Nucl Chem 287, 567–572 (2011). https://doi.org/10.1007/s10967-010-0774-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0774-3