Abstract
The preparation and characterization of three different phases of zirconium phosphate (ZrP) have been carried out using XRD, SEM, EDX, and FTIR spectroscopy. The phases exchange and sorption properties with three different radioisotope 234Th, 238U, and 134Cs were investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ion exchange technologies are widely applied for water and waste treatment in different areas of research and industry such as hydrometallurgy, biochemistry, medicine, and environmental protection. Ion exchange as a separation process is relatively easy and energy-efficient in comparison with other traditional waste treatment techniques used in nuclear industry (e.g., solvent extraction, precipitation, evaporation…). The nuclear industry produces large volumes of radioactive waste which requires treatment prior to final disposal. This requirement can be met with the use of inorganic ion exchangers.
Ion exchange efficiency depends on many factors, the main principal one being the selectivity of the exchanger in use. Inorganic ion exchangers and adsorbent materials are very important due to their chemical thermal, stability, and resist to oxidation whereas unique selectivity to certain ions has definite advantages in comparison with other traditionally used organic resins.
Zirconium phosphate (ZrP) has recently been demonstrated as an excellent sorbent for heavy metals due to its high selectivity, high thermal stability, and absolute insolubility in water [1–3].
ZrPs can be exist in amorphous or crystalline phases and have a general formula ZrO2·nP2O5·mH2O·(xMO), where n = 0–2.5, m > 0, x = 0–5, M = metal ion.
Conventional methods of preparing amorphous ZrP can be achieved the reaction between aqueous solutions of a zirconium salt and a phosphorus containing reagent, such as phosphoric acid or its salts [4–7]. Crystalline ZrP can be prepared by treatment of amorphous ZrPs in the presence of excess phosphoric acid at elevated temperature for a long period of time [8] or by a reaction between aqueous solutions of a zirconium salt and phosphoric acid to form a gel and then heating the dry gel in water under hydrothermal conditions [9] or also via solid state reactions between ZrO2 or zirconium salts and salts of phosphoric acid [6, 10].
The objective of the present study is the preparation and characterization of ZrP in order to explore the behavior and the mechanism of radionuclide sorption onto amorphous, anhydrous crystalline and monohydrate crystalline phases of ZrP, as well as, the sorption with three radioisotopes 234Th, 238U, and 134Cs ions elements in aqueous solution.
Experimental
Materials and instrumentation
The formation and characterization of ZrP were identified using different techniques: X-ray diffraction (XRD), the data were collected with a Stoe StadiP Transmission X-ray diffractometer. Infrared Fourier transform (FT-IR) spectra were taken in Thermo Nicolet 6700 spectrophotometer with a resolution of 4 cm−1 as KBr disc. Approximate particle size determinations ZrP samples were obtained with a Tescan Vega II XMU Scanning Electron Microscope (SEM). Energy Dispersive X-ray Spectroscopy (EDX) is used in parallel with SEM for micro analysis determination of the prepared samples.
Gamma spectroscopy and fluorometry measurements were used for detection and determination of the studied radioisotopes, respectively. Thorium (234Th, γ, 63 kev, 92 kev) was separated from the natural uranium series using the method described earlier [11]. Uranium (238U, α) was separated from the natural uranium series by dissolving 1 g of U3O8 in 10 mL HNO3 (8 M), then heated to dryness and followed by dissolving in HCI (8 M), then passed through an anion exchange resin for fixing uranium. The uranium was finally extracted by resin elution using a diluted HCl 0.1 M. Cesium (134Cs, γ, 606 kev, 797 kev) was obtained by irradiating of 0.1 g of natural cesium at the Miniature Neutron Source Reactor of Syria, MNSR reactor at 5 × 1011 n/s cm2 flux.
Preparation of ZrPs
Three ZrPs phases were prepared, which were classified and identified according to the preparation conditions. The first phase, ZrP1 is the monohydrate crystalline with a formula of Zr(HPO4)2·H2O. The second phase ZrP2, with the form of Zr(HPO4)2, is amorphous in nature as elucidated elsewhere [7, 12], while the third and last phase, ZrP3 is the crystalline anhydrous form with the formula of ZrP2O7.
ZrP1
The monohydrate crystalline phase (ZrP1) was prepared applying the method proposed by Bauer and Helen [13, 14]. 25 mL aqueous solution of zirconyl chloride (ZrOCl2·8H2O, 1 M) was slowly added to ten times excess of 1 M H3PO4 (about 250 mL). The precipitated material was washed several times with distilled water to remove the un-reacted materials and impurities, then dried for 2–3 h at 95 °C and finally stored at room temperature.
ZrP2
Preparation of amorphous phase (ZrP2) has been carried out as follows: 10 g of ZrOCl2·8H2O was first dissolved into 20 mL 2 M HCl solution. At the ambient temperature, the above solution was gradually added into a flask containing 180 mL phosphoric acid with a concentration of 3.5 M within 30 min and shaken overnight at 120 rpm. Thereafter, the precipitate (ZrP) was filtered, and then rinsed first with about 500 mL 0.5 M H3PO4 solution and finally washed by distilled water until the neutral pH is obtained. The obtained particles, ZrP particles were desiccated at 50 °C for 24 h [2, 8] and stored at room temperature for further work.
ZrP3
The crystalline anhydrous phase (ZrP3) was prepared by two procedures:
-
(a)
17 g phosphoric acid (9 M) was added to excess of 10 g ZrOCl2·8H2O (31 mmol). The reaction mixture was heated at 90 °C for 24 h. The resultant white product was washed twice with 50 mL distilled water and one time with 20 mL of acetone. The obtained solid material was dried overnight at 60 °C [8].
-
(b)
5 g ZrCl4 was dissolved in 30 mL HCl (2 M) and stirred at room temperature, then 3 mL phosphoric acid (2.3 M) was added to the stirred mixture. The produced gel material was then refluxed in H3PO4 (about 3.3 M) until crystalline ZrP was formed. The precipitated solid was washed with H3PO4 (0.7 M) and then with distilled water and the pH was adjusted to 4 in order to get rid of all un-reacted phosphate and chloride ions. Finally the prepared compounds was dried at 70 °C for 72 h [15].
Weight exchange capacity
To determine the total weight exchange capacity of ZrP, 1 g of the previous air-dried exchanger is swollen with distilled water. The swollen resin was transferred to an ion exchange column. Amount 200 mL of 0.1 M NaOH was passed through the resin bed at a flow rate of 2–3 mL/min. After the total amount of NaOH solution has passed through the column, the residual liquid present in the column was ejected with compressed air. The loss of OH− ions was determined by titration with 0.1 M HCI, and the total weight exchange capacity, expressed in millimoles of H+ per gram (mmol/g) of dry exchanger, was calculated as mentioned in literature [16].
Results and discussion
Characterization
ZrP1 and ZrP2 were obtained as white hard granules materials while the ZrP3 was formed as white powder.
The sharp peaks in the XRD pattern of ZrP1 (Fig. 1) correspond to the formula Zr(HPO4)2·H2O [17, 18]. Figure 2 shows three different FTIR spectra, The IR spectrum of Zrp1 shows a broad band observed at 3400 cm−1 and another band at 2360 cm−1. These two bands are corresponding to O–H (hydroxyl group and water molecule) and POH stretching vibrations, respectively. The peak at 650 cm−1 is connected with the O–H bond (out of plane). The bands at 506 and 1080 cm−1 are assigned to Zr–O and PO4 asymmetric stretching vibrations which are the most characteristics modes of ZrP1 [14, 19, 20].
The absence of any sharp peaks in the X-ray powder pattern (Fig. 1) for ZrP2 indicates the amorphous nature of the material [7, 12]. The FTIR spectrum of ZrP2 (Fig. 2) exhibits a broad band in the region around 3400 cm−1 which is attributed to –OH stretching band. A sharp with a medium intensity band at 1635 cm−1 is assigned to (H–O–H) bending band. The band at 1080 cm−1 is confirms the formation of P=O stretching. fundamental bonds presented in Zr(HPO4)2 [2, 21].
The sharp peaks in the XRD for ZrP3 (Fig. 1) indicates clearly the crystalline nature of this phase and were correspond the formula ZrP2O7 [22–24]. In the absence of hydrogen in this crystalline phase leading to the disappearance of band observed previously at 2360 cm−1 (POH stretching mode). Also, the O–H stretching band at 3400 cm−1 shifted to lower frequency and it is narrower than the bands observed in the two previous phases (Fig. 2). This observed IR result is consistence with XRD observations confirming the existence of anhydrous crystalline phase (ZrP3).
The SEM results are compatible with the previously observed XRD and IR data. The micrography images of ZrP are shown in Fig. 3, which shows clearly the presence of the three phases of ZrP (ZrP1: monohydrate crystals, ZrP2: amorphous, ZrP3: anhydrous crystals). On the other hand, the oxygen, phosphorus, and zirconium elemental contents of the surfaces for three phases were determined using EDX technique. Table 1 summarizes the theoretical and measured values for above mentioned elements for the three investigated phases. The reported data in Table 1 for O/P, O/Zr, and P/Zr ratios between theoretical and measured values are very close to each other. This emphasis that the three obtained phases has the suggested formula as pointed out in this work.
Sorption studies
The Na+ weight exchange capacity of ZrP at room temperature was determined using an acid–base titration method. The weight exchange capacity results for the different phases are given in Table 2. The weight exchange capacity is in the following order: ZrP1 > ZrP2 > ZrP3.
Six standard (Merck) aqueous solutions with different concentrations (acidic or basic milieus) were used for the preparation of radioisotope solution as follows: tartaric acid (0.01–3 M), citric acid (0.01–3 M), HCl (0.1–8 M), HNO3 (0.1–8 M), NaOH (0.1–8 M), and NH4OH (0.1–8 M).
The sorption of radioisotopes on these three phases of ZrPs (three forms) was investigated as follows; 20 mL from each last six standard aqueous solution containing one of the following radioisotope 234Th (1000 cps), 238U (75 ppm), and 134Cs (500 cps) was added to 0.5 g ZrP and left for 24 h for sufficient ionic exchanging. It should be mentioned here that, the experiments were repeated three times with each individual prepared ZrP phases. By the way, the radioactivity was measured before and after mixing with ZrP.
Thorium
The sorption behavior of 234Th on ZrP is varying from a solution to another and it depends on the ZrP phase. The ZrP1 is excellent exchanger for 234Th because the sorption was fully in HCl, HNO3, NaOH, and NH4OH solutions. Nevertheless, at low concentrations of tartaric acid and citric acid (less than 0.1 M), the 234Th sorption is lower (between 80 and 95%).
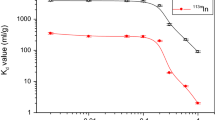
In ZrP2 and ZrP3, the sorption of 234Th in HCl, NaOH, tartaric acid, and citric acid milieus was fully. While in NH4OH solution, the sorption was lowered to 80%. The kinetic behavior of 234Th sorption on ZrP phases in HNO3 solution is illustrated in Fig. 4. As can be seen in Fig. 4, 234Th sorption on ZrP2 increases with increasing HNO3 concentration. On the contrary, the sorption on ZrP3 decreases with increasing concentration of HNO3. But, it should be pointed, that the sorption is complete with ZrP1 phase. The obtained results can be explained on the following bases: considering that the mechanisms of sorption for ZrP1 phase are a accompanying of the two sorption mechanisms for ZrP2 and ZrP3 phases, which will be discussed later. Therefore, the sorption of 234Th on ZrP1 would be the sum of the ZrP2 and ZrP3 curves (Fig. 4).
Uranium
It has been found that the sorption of 238U on ZrP is lower than 234Th in all aqueous solutions for the three different phases of ZrP. In all acid solutions, the sorption of uranium on ZrP decreases with increasing acid concentrations. The 238U sorption is worse in the HNO3 solution, it does not exceed 60% at best (0.3 M). In HCl solution (Fig. 5), the percentage sorption is better than HNO3 solution with, 85% yield. But the best sorption of uranium is in citric acid (Fig. 6) and tartaric acid (Fig. 7), where the sorption is more than 90%. This result is agreeable with the changes of surface properties produced by citric acid during the hydration process [25]. It can be concluded that the best and worst uranium sorption will be with ZrP2 an ZrP3 phases, respectively. Moreover, the sorption on ZrP3 becomes better in the presence of organic acids with ZrP2O7 due to increase of uranium sorption constants [25].
Cesium
Generally in acid solutions, the sorption of 134Cs on ZrP1 and ZrP2 phases was nearly fully but with ZrP3, the 134Cs sorption increases with increasing acid concentration (Fig. 8).
In HCl solution, the rate of sorption was less than 60 and 100% for concentrations lower and higher than 1 M, respectively. In HNO3, tartaric acid and citric acid solutions, the sorption of 134Cs is about 80%.
For alkaline solutions, the sorption of 134Cs with NaOH on ZrP1 phase was between 84 and 100% with all investigated concentrations (Table 3). Regarding ZrP2 phase, the sorption of 134Cs is less than previous phase (about 56% with 0.5 M NaOH). Finally, the sorption of 134Cs with NaOH on ZrP3 phase is lower for concentrations lower than 1 M in particular (about 30% with 0.8 M NaOH).
With NH4OH, the best 134Cs sorption was with ZrP2 phase (Table 4). Similarly, Parker et al. found that amorphous ZrP can selectively remove Cesium even in the presence of a competitive ion of high concentration [1]. As 100% of cesium sorption is obtained by ZrP, it can be concluded that this method is competitive with electrochemical ion exchange technique [26].
It can be concluded that the differences in the behavior of ZrP phases are related to the structures and the compositions for these phases. Moreover, the differences between the ion exchange properties of crystalline phases (ZrP1 and ZrP3) could be explained by the building of different structural arrangements [27].
The sorption could be explained by the two following mechanisms. The first mechanism is ion exchange, while the second mechanism is ions adsorption on the surface. In the amorphous phase ZrP2, the ion exchange mechanism is dominant and can be represented by the following equation [2]:
where M represents the corresponding element of heavy metal.
For anhydrous crystalline phase ZrP3, in absence of the hydrogen atoms (which play main role in the ion exchange mechanism), the adsorption mechanism is dominant. This could be explained by the formation of active sites in solid/liquid interface due to the hydration of ZrP2O7 [25].
Finally, for monohydrate crystalline phase ZrP1, adsorption mechanism occurs on the surface, as well as the ion exchange mechanism, see the following equation [5]:
where M is a metal and n is a number (M = Na when n = 5).
This interpretation is compatible with the previous mentioned results of the exchange capacity and sorption, where generally the ZrP1 phase is the best active one while the ZrP3 phase is the least active.
On the other hand, the sorption could become better using the ZrPs in saline form, due to its better sorption capacity for heavy polyvalent metals [28].
Conclusion
Three phases of ZrPs were prepared by adding zirconyl chloride to phosphoric acid at different conditions. The samples were characterized by XRD, SEM, EDX, and FTIR spectroscopy. Ion exchange and sorption behavior was investigated using 234Th, 238U, and 134Cs as radioisotopes in aqueous solutions and with different acid and bases mediums.
The best sorption of thorium was found to be with ZrP1 phase apart from solutions concentrations. In addition to that, the ZrP1 is considered to be the best used phase for cesium sorption. The best sorption of Uranium was with ZrP2 phase in the tartaric acid with a concentration less than 1 M.
It is suggested that, the ZrP can be used as ion exchange resins. All the three prepared phases of ZrP have different exchange properties. It is emphasized also that ZrP phases can be used for actinides separation in aqueous solution. For example a method to separate thorium from uranium using ZrP1 phase in 3 M HNO3 solution could be proposed according to obtained in this article (the sorption of thorium was complete, while the uranium sorption was less than 10%).
References
Park H-S, Kim I-T, Kim H-Y, Ryu S-K, Kim J-H (2006) Immobilization of molten salt waste into MZr2(PO4)3 (M = Li, Na, Cs, Sr). J Radioanal Nucl Chem 268(3):617–626
Pan B, Zhang Q, Du W, Zhang W, Pan B, Zhang Q, Xu Z, Zhang Q (2007) Selective heavy metals removal from waters by amorphous zirconium phosphate: behavior and mechanism. Water Res 41:3103
Zhang QR, Du W, Pan BC, Pan BJ, Zhang WM, Zhang QJ, Xu ZW, Zhang QX (2008) A comparative study on Pb2+, Zn2+ and Cd2+ sorption onto zirconium phosphate supported by a cation exchanger. J Hazard Mater 152(2):469–475
Amphlett CB (1964) Inorganic ion exchangers. Elsevier, New York
Clearfield A (1982) Inorganic ion exchange materials. CRC Press, Inc, Boca Raton
Patel HK, Josh RS, Chudasama UV (2008) Use of zirconium(lV) phosphate as a solid acid catalyst in some esterification reactions. Indian J Chem 47A:348–352
Zhao GL, Yuan ZY, Chen TH (2005) Synthesis of amorphous supermicroporous zirconium phosphate materials by nonionic surfactant templating. Mater Res Bull 40:1922–1928
Clearfield A, Stynes JA (1964) The preparation of crystalline zirconium phosphate and some observations on it ion exchange behavior. J Inorg Nucl Chem 26:117–129
Dongare MK, Singh P, Suryavanshi PM (1992) Hydrothermal synthesis and characterisation of crystalline sodium zirconium phosphates. Mater Res Bull 27(5):637–645
Winand JM, Rulmont A, Tarte P (1993) Synthesis and study of new compounds (MI) (NIV)2(PO4)3 with nasicon-like structure (M = Ag, Cu; N = Ge, Hf, Sn, Ti, Zr). J Solid State Chem 107(2):356–361
Alhassanieh O, Abdul-Hadi A, Ghafar M, Aba A (1999) Separation of Th, U, Pa, Ra and Ac from natural uranium and thorium series. Appl Radiat Isot 51(5):493–498
Trobajo C, Khainakov SA, Espina A, Garcı′ JR (2000) On the synthesis of r-zirconium phosphate. Chem Mater 12:1787–1790
Bauer F, Willert-Porada M (2005) Characterisation of zirconium and titanium phosphates and direct methanol fuel cell (DMFC) performance of functionally graded Nafion(R) composite membranes prepared out of them. J Power Sources 145:101–107
Helen M, Viswanathan B, Murthy SS (2007) Synthesis and characterization of composite membranes based on α-zirconium phosphate and silicotungstic acid. J Memb Sci 292:98–105
Asghari FS, Yoshida H (2006) Dehydration of fructose to 5-hydroxymethylfurfural in sub-critical water over heterogeneous zirconium phosphate catalysts. Carbohydr Res 341:2379–2387
Korkisch J (2000) Handbook of ion exchange resins: their application to inorganic analytical chemistry, vol 1. CRC Press, Inc., Boca Raton
Ahrland S, Albertsson J (1969) Inorganic ion exchangers. VI. The unit-cell dimensions of crystalline zirconium phosphate. Acta Chem Scand 23(4):1446
Clearfield A, Smith GD (1969) Crystallography and structure of α-zirconium bis(monohydrogen orthophosphate) monohydrate. Inorg Chem 8(3):431–436
Horsley SE, Nowell DV, Stewart DT (1974) The infrared and Raman spectra of α-zirconium phosphate. Spectrochim Acta 30A:535
Rajeh AO, Szirtes L (1999) FT-IR studies on intercalates and organic derivatives of crystalline (α- and γ-forms) zirconium phosphate and zirconium phosphate-phosphite. J Radioanal Nucl Chem 241(1):83–91
Thakkar R, Patel H, Chudasama U (2007) A comparative study of proton transport properties of zirconium phosphate and its metal exchanged phases. Bull Mater Sci 30(3):205–209
Chaunac M (1971) Etude cristallographique du pyrophosphate de zirconium. Bull Soc Chim Fr 2:424–429
Hagman L-O, Kierkegaard P (1969) Note on the structures of MIV P2O7 (MIV = Ge, Zr, and U). Acta Chem Scand 23:327
Huang C-H, Knop O, Othen DA, Woodhams FWD, Howie RA (1975) Pyrophosphates of tetravalent elements and a Mössbauer study of SnP2O7. Can J Chem 53(1):79–91
Garcia-Gonzalez N, Ordóñez-Regil E, Simoni E, Barrera-Díaz C (2010) Effect of organic acids on sorption of uranyl ions in solution onto ZrP2O7. J Radioanal Nucl Chem 283(2):409–415
Manosso H, Forbicini C (2009) Treatment of wastes containing cesium ions by electrochemical ion-exchange (EIX). J Radioanal Nucl Chem 279(2):417–422
Nilchi A, Maragheh MG, Khanchi A, Farajzadeh MA, Aghaei AA (2004) Synthesis and ion-exchange properties of crystalline titanium and zirconium phosphates. J Radioanal Nucl Chem 261(2):393–400
Zhuravlev I, Zakutevsky O, Psareva T, Kanibolotsky V, Strelko V, Taffet M, Gallios G (2002) Uranium sorption on amorphous titanium and zirconium phosphates modified by Al3+ or Fe3+ ions. J Radioanal Nucl Chem 254(1):85–89
Acknowledgments
The authors like to thank Prof. Dr. I. Othman the Director General of the Atomic Energy Commission of Syria and Prof. Dr. T. Yassin (head of chemistry department) for their encouragement. The authors would like to thanks Dr. M. Rukiah for fruitful discussions with XRD results and Prof. Abdul Wahab Allaf for his discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mrad, O., Abdul-Hadi, A. & Arsan, H. Preparation and characterization of three different phases of zirconium phosphate: study of the sorption of 234Th, 238U, and 134Cs. J Radioanal Nucl Chem 287, 177–183 (2011). https://doi.org/10.1007/s10967-010-0679-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0679-1