Abstract
This study was conducted to investigate the effect of time on cadmium (109Cd) availability in four typical soils of the Danubian Lowland through the modified Tessier’s sequential extraction procedure as well as its short-term sorption in the bulk soils and their two grain-size fractions. Results of the fractionation study showed that there were significant changes in the proportional distribution of cadmium in all studied soils during 180 days of incubation with spiked cadmium. Generally, the proportions of cadmium associated with the most weakly bound fractions (water soluble and exchangeable) tended to decrease with corresponding increases in the residual fraction during the incubation. The extent of cadmium sorption in all studied soils was high, exceeding 95% of the spiked amount after 60 min of incubation, likely due to slightly alkaline character of the soils. The finding that soil particles less than 10 μm sorbed up to 51% of the spiked cadmium in soils is of great importance since they could play a role in colloid-facilitated transport of cadmium through preferential pathways, as previously observed in the region. Addition of 1 M ammonium nitrate into the soil solution generally decreased cadmium sorption in all four soils. The lowest extractabilities of Cd were obtained using 1 M ammonium nitrate as a single extractant, whereas 0.025 M ammonium ethylenediaminetetraacetate solution extracted the highest proportions of cadmium from the studied soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the most common heavy metal in the soil environment and its concentrations have increased seriously during the last few decades due to the agricultural application of sewage sludge, phosphate fertilizers, fungicides and land disposal of metal-contaminated municipal and industrial wastes [1–3]. Cadmium is a soil pollutant of no known essential biological functions, and may pose threats to soil-dwelling organisms and human health [4, 5]. Cadmium present in soils may accumulate in food crops or may leach through soils polluting groundwater supplies. Thus concerns about soil and water pollution have emphasized the importance of understanding the processes or factors, which control the retention and release of Cd in soils.
The concentration of Cd in the soil solution, and hence its downward transport, bioavailability and toxicity is controlled mainly by sorption–desorption processes at the surfaces of both inorganic and organic soil colloids. It is well-known that pH is the most important soil parameter determining the mobility of Cd in soils, which decreases universally with increasing soil pH [6–9]. However, other factors such as soil organic carbon content, cation exchange capacity, clay content, content of Fe and Mn oxyhydroxides and solution composition have a pronounced effect on the retention and mobility of Cd in soils [10–12]. Although downward movement of Cd in soils is largely controlled by its concentration dissolved in the soil water, colloid-facilitated transport via preferential pathways may result in a deeper penetration of Cd in soils, i.e. much more as it might be predicted based only on its sorption characteristics in bulk soils. Lichner [13] showed in a field experiment that Cd penetrated up to the depth of 65 cm in soils of the Danubian Lowland, the phenomenon, which could not be explained by single-continuum model simulating the movement of solutes in the soil matrix. Vogel et al. [14] suggested that colloid-facilitated transport by soil particles less than 10 μ m, which sorbed a substantial proportion of added Cd, were responsible for the observed deep penetration of Cd in soils of the Danubian Lowland.
Total cadmium contents are of limited importance in evaluating its mobility and bioavailability in soils. The mobility and bioavailability of cadmium depend strongly on its chemical forms associated with inorganic and organic soil components. Various analytical approaches have been used for the determination of different chemical forms of Cd, and many of them rely on the element desorption from the solid phase using chemical reagents. Two groups of tests should be considered: (i) the single extraction procedures using one extraction reagent, and (ii) the sequential extraction procedures using a series of progressively harsher reagents to dissolve increasingly refractory forms [15–17]. Single extraction reagents can be divided into three main classes: weak replacement of ion salts, dilute solutions of either weak or strong acids and chelating agents [15, 18]. Sequential extraction procedures have also been used to investigate the effect of contact time on the fractionation of freshly spiked heavy metals in soils [19–21]. Contact time directly relates to the bioavailability and toxicity of heavy metal in soils. Studies focused on the temporal changes in the fractionation and bioavailability of heavy metals have shown that their bioavailability decreases and they are converted from easily available and soluble forms into less soluble ones with increasing contact time [19, 22–24].
The aim of this study was to investigate the effect of contact time on the fractionation of added Cd in four agricultural soils of the Danubian Lowland (south-west Slovakia). Moreover, the short-term sorption of cadmium chloride solution labeled with 109Cd in bulk soils and their two grain-size fractions, and the effect of ammonium cations on the Cd sorption were evaluated in this study. Finally, three single extraction reagents, widely employed to assess the bioavailability of heavy metals in soils, were used to extract sorbed Cd after 1 day of incubation and to compare the Cd proportions extracted by single reagents with those obtained by the modified Tessier’s sequential extraction procedure.
Experimental
Soil samples
This study was conducted with light, medium heavy, and heavy soils of the Danubian Lowland, which is a large (1260 km2) agriculturally managed area situated in the south-west Slovakia, with a shallow (0.5–3.8 m deep) underlying aquifer containing about 10 km3 of groundwater with the high quality. The light soil (loamy-sand soil, Calcaric Fluvisol), the two medium heavy soils (loamy soil, Calcaric-Haplic Chernozem and clay-loam soil, Calcaric Chernozem) and the heavy soil (clay soil, Calcaric-Mollic Fluvisol) were taken from Kalinkovo, Macov, Most and Jurová, respectively [25]. Selected physico-chemical properties of the topsoil layers (0.1–0.2 m) are shown in Table 1 [26, 27]. Quality of soil humus was assessed as the ratio of humic acid to fulvic acid contents (HA/FA).
Sequential extraction procedure
For the incubation study, duplicate 40 cm3 aliquots of water solution containing radioactive 109Cd (in the form of CdCl2) with a concentration of 50.9 mg dm−3 were added to 10 g of dry soil (<2 mm) in 100 cm3 polyethylene bottle. The soil suspensions were incubated for 1, 30, 90, and 180 days at 20 °C. After incubation, soil suspensions were centrifuged for 5 min at 5,000 rpm, the aqueous phase was removed as much as possible, and consequently a modification of the Tessier’s sequential extraction procedure [28] was used to extract cadmium from amended soils. Individual steps of the modified sequential extraction procedure applied to the soils are given in Table 2. At the end of each extraction step, the samples were centrifuged for 5 min at 5,000 rpm to separate the soil. The soil was washed with distilled water and again centrifuged. The washed water was discarded. Activity of 109Cd in the extracts was measured multi-channel gamma-spectrometer (Series 35 Plus, Canberra Industries Inc., Meriden, CT, USA) with Ge/Li detector.
Single extractions
To assess the cadmium individual forms in soils, the soil samples spiked with cadmium were prepared as described above. After 1 day of incubation, the samples were centrifuged for 5 min at 5,000 rpm, the aqueous phase was carefully removed, and then, 40 cm3 of single extractants, i.e. 1 M ammonium nitrate (NH4NO3), 2 M nitric acid (HNO3) and 0.025 M ammonium ethylenediaminetetraacetate (EDTA) were added to the soil samples. The soil suspensions were shaken for 1 h, centrifuged and analyzed. It is generally reported that the exchangeable Cd, usually determined by unbuffered weak salt solutions such as NH4NO3, represents the most mobile fraction of soil and readily available for plant uptake [15, 29]. Nitric acid-extractable metals in the soil have been suggested as a measure for geochemically active metals, present in the soil [30]. The term active implies susceptibility to chemical interactions with the soil solids that control solution concentrations. However, this proposed terminology may be somewhat vague, and the fraction may therefore best be referred to as acid-extractable metals. Complexing extractants such as EDTA or DTPA can, by virtue of their strong complexing ability, displace metals from insoluble organic or organometallic complexes in addition to those sorbed on inorganic soil components [31, 32].
Sorption experiments
The sorption of cadmium in bulk soils was determined by the conventional batch technique. Each sorption experiment involved 10 g of dry soil passed through a 2 mm sieve before use, 40 cm3 of distilled water, and radioactive cadmium (109Cd in the form of CdCl2) with a concentration of 50.9 mg dm−3 and specific activity a 0. Soil, water and cadmium solution were placed into a 100 cm3 polyethylene bottle and shaken vigorously for 5 s. Then, a 5 cm3 of the soil suspension was taken at t = 1 min after shaking, centrifuged, and the specific activity a(t) of 109Cd in the aqueous phase was measured using a multi-channel gamma-spectrometer (Series 35 Plus, Canberra Industries Inc., Meriden, CT, USA) with Ge/Li detector. The proportion of cadmium sorbed in bulk soils at a given time was calculated from the equation:
The same procedure was used for the Cd–soil contact times t of 2, 3, 5, 10, 30, and 60 min.
The sorption of cadmium by soil particles less than 10 μm was determined by the modified batch technique with radioactive cadmium 109Cd and no centrifugation. Sorption experiments were conducted as described above for bulk soils, but the soil suspension taken at 1 min after shaking was not centrifuged. Thus, the measured specific activity [denoted as a′(t)] corresponded to 109Cd in the aqueous phase and sorbed in the soil particles <10 μm. The proportion of Cd sorbed in soil particles larger than 10 μm at a given time was calculated as follows:
and the proportion of Cd retained by soil particles less than 10 micrograms could be calculated using the following equation:
The same procedure was chosen for the Cd–soil contact times of 2, 3, 5, 10, 30, and 60 min, but with the modification that in the final phase of contact (=65 s before its finishing) the soil suspension was shaken for 5 s and then sampled after 1 min. This selected time (t = 1 min) was evaluated by the Stokes law, according to which the soil particles smaller than 10 μm had not enough time to settle, and hence remained suspended in the aqueous phase. The proportions of cadmium sorbed in bulk soils and soil particles larger than 10 μm were used to calculate the distribution coefficients for matrix and preferential flow domains, respectively, in two-domain cadmium transport modelling [33, 34].
The effect of ammonium cations on Cd sorption in bulk soils was evaluated by the conventional batch technique described above using a 1 M NH4NO3 solution.
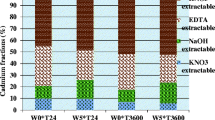
All the sequential extractions, single extractions and sorption experiments were made in duplicate and both the measured values and means are presented in Figs. 1, 2, 3 and 4.
Results and discussion
Characterization of soils
Selected chemical and physical properties of the soils are shown in Table 1. Clay contents ranged from 11.5 to 23.0%, total organic carbon contents ranged from 0.78 to 2.20%, and contents of soil particles smaller than 10 μm were from 21.6 to 51.8%. The soils were loamy sand to clay in texture, and exhibited alkaline character, which could be attributed to the relatively high calcium carbonate contents, varying from 16 to 27%. The clay fraction of all studied soils was composed mainly of illite, followed by chlorite, smectite, calcite, dolomite and quartz [26]. The highest HA/FA ratio had the clay soil from Jurová, indicating a good quality of soil humus in this soil.
Temporal changes in the fractionation of Cd
The effect of contact time on the distribution of Cd between different soil fractions is shown in Fig. 1. The results presented in Fig. 1 clearly show that water soluble Cd decreased markedly within 30 days, remained relatively constant with increasing contact time, and were approximately identical for all soils at the end of incubation study. As could be seen from Fig. 1, most of Cd added to soils appeared in the exchangeable fraction after 1 day of incubation. Cadmium in the exchangeable fraction was 39.4, 29.3, 20.3 and 32% in Kalinkovo, Macov, Jurová and Most soils after 1 day of incubation. When the contact time increased, proportions of added Cd in the exchangeable fraction decreased gradually and were between 10–13% at day 180. The proportions of carbonate-bound Cd also decreased to some extent during the incubation (Fig. 1), although the rates at which the changes occurred with the proportion of carbonate-bound Cd appeared to be much lower compared to the rates associated with the exchangeable Cd fractions. Organic-bound Cd was found to increase when contact time increased from 1 to 30 days in all soils, but then generally decreased and the proportions of Cd bound to organic matter were significantly lower at day 180 compared to those at day 1 (Fig. 1). The amounts of Cd fraction dissolved in mineral acid decreased continually during the incubation study, but with the different rates, when the highest decrease in the proportions of Cd dissolved in mineral acid occurred between 30 and 60 days. No obvious trends were observed for Cd associated with Fe/Mn oxides and dissolved in sodium hydroxide (Fig. 1). Cadmium that was added to the soils transformed mainly to the less soluble forms as reflected by the continual increase in the proportions of Cd associated with residual fraction during the incubation. It could be concluded that there were clear changes in the fractionation of Cd in all four studied soils during the whole period of incubation. The main outcome of this study is that the amounts of water soluble and exchangeable Cd, which are considered as the most bioavailable and susceptible forms for downward leaching in soils, tended to decrease with time, whereas the amounts of Cd in the most strongly bound fractions (i.e. residual) clearly increased indicating a decline in its bioavailability and hence potential toxicity to animals and humans. Since the soils have similar characteristics concerning, e.g. their pH values and calcium carbonate contents, there were no significant differences in the rates at which redistribution of Cd took place during the incubation.
Despite the differences in sequential extraction procedures used, the results of this study were in close agreement with the findings of previous studies. Mann and Ritchie [35] investigated the changes in the forms of added Cd through eight days in soils, and found that the most labile fractions of Cd (water soluble and exchangeable) transformed with time to least available and soluble forms (residual). In another study, Tang et al. [36] also observed that the added Cd in the exchangeable fraction of five soils decreased with time (through 120 days), with corresponding increases in the proportions of Cd associated with residual fraction.
Single extractions
The extractabilities of spiked Cd after 1 day of incubation using single extractants are depicted in Fig. 2a. The NH4NO3, HNO3, and EDTA solutions used to extract the soils demonstrated variability in capacity to extract Cd from the soils and the following order of their increasing extraction capacity was observed: NH4NO3 < HNO3 < EDTA. It is interesting to compare the proportions of Cd extracted by NH4NO3 with those extracted by MgCl2 in the modified Tessier’s sequential extraction procedure. As could be seen from Figs. 1 and 2a, the MgCl2 solution was able to extract the much higher amounts of Cd from all soils than the NH4NO3 solution did. This was likely due to different cation competitiveness for adsorption to the soil, and therefore in the ability to release competing cations such as Cd from the soil matrix. Generally, the following order of competitiveness of exchangeable cations used in single extraction procedures has been observed: Ba > Ca > Mg > NH4 > K > Na [37]. The total pool of potentially available metal species in soils can be estimated from the EDTA-extractable fraction [1]. EDTA, as a strong chelating agent, is considered to extract metals in all the non-silicate-bound soil phase, i.e. is capable of desorbing not only the mobile forms of the metals (water soluble and exchangeable), but also those more strongly bound to organic and inorganic non-silicate soil constituents [32]. In this regard, the proportions of Cd extracted by this single procedure were comparable to the sum of the proportions of Cd extracted in the first six steps of the modified Tessier’s procedure, as shown in Fig. 2b.
Sorption of Cd in soils
The sorption of Cd and other elements by solids was commonly investigated as a function of contact time in previous experiments and the results showed that the sorption was very fast and equilibrium was reached almost instantaneously after mixing [38–42]. The relative proportions of Cd sorbed in bulk soils with time are shown in Fig. 3. It could be seen that the sorption of Cd was rapid and very efficient, exceeding 95% of the total Cd added to soils after 1 min. The observed short-term kinetics of Cd sorption satisfied well the pseudo-second order kinetic equation, which can be written in its linear form as [43, 44]:
where k 2 is the pseudo-second order rate constant. This likely indicated that the rate of direct sorption process (seen as a kind of chemical reaction) controlled the overall sorption kinetics of Cd in the soils. The extent of Cd sorption after 60 min in bulk soils, expressed as percentage of the total added Cd was very high with only minor differences among the soils, and followed the order: Kalinkovo (99.8%) > Jurova (99.2%) = Most (99.2%) > Macov (97.2%). The high sorption capacity of the soils for Cd might be due to their neutral up to slightly alkaline soil pH, high calcium carbonate, silt and clay contents. A dominant role of clays, carbonates and soil pH for the sorption of Cd in soils was confirmed previously by Hooda and Alloway [10], Adhikari and Singh [7], Palágyi and Rigas [39], and more recently by Shaheen [12]. The sorption results with soils of the Danubian Lowland showed that Cd added to the soils might be retained efficiently, and hence they might reduce the potential of Cd to leach via the soil matrix into groundwater.
The results of the modified batch technique revealed that significant proportions of Cd were adsorbed by the soil particles smaller than 10 μm within 1 min of incubation (Table 3). However, during the incubation, cadmium was quickly re-distributed between the soil particles <10 μm and the particles >10 μm, reaching an equilibrium state after 30 min. Decrease in the extent of Cd sorption by soil particles less than 10 μm was balanced by increase in the Cd sorption by soil particles larger than 10 μm. The observed particle-size dependent and temporal changes of Cd distribution in all studied soils remain poorly understood, but might be related with the corresponding changes in degree of aggregation of the soil particles with increasing time. Nevertheless, the results confirmed that the soil particles less than 10 μm sorbed a substantial part of Cd, and these particles might transport cadmium towards groundwater through preferential pathways, i.e. macropores, since the hydrogeological setting of the soil sites is dominated by macropore flow and less by the flow in the low permeability matrix [45]. The quantitative impact of such transport was not evaluated in the present study, but previously, Vogel et al. [14] confirmed by the field transport experiment at the studied site and the dual-continuum modelling that more than 40% of the applied Cd was transported deeper than 10 cm through macropore-mediated transport of Cd in particle-bound form and liquid phase.
The presence of NH4 + cation in the aqueous solution, as a common component of fertilizers, reduced slightly total Cd sorption in soils as compared to its absence (Fig. 4). The maximum reduction of Cd sorption by 3.3% in the presence of NH4 + was observed for the soil from Kalinkovo, which had the highest exchangeable proportion of Cd among the studied soils (Fig. 1). The inhibitory effect of NH4 + on soil sorption was previously reported for Zn, an element very familiar to Cd, and this was attributed to direct competition for adsorption sites and effect of NH4 + on surface negative charge density [46]. He et al. [47] reported that the use of NH4 + also enhanced the release of Zn from Florida sandy soils with exchangeable Zn >23% as compared to water and pointed out the role of cation exchange competition between Zn and NH4 + in the electrolyte.
Conclusion
There is a need for studying cadmium distribution and retention in soils since groundwater contamination and uptake by plants depends strongly on these processes. In this study, four calcareous soils of the Danubian Lowland (Slovakia), the area with highly vulnerable groundwater resources, were used to investigate the fractionation of added Cd over time, its sorption and the effect of ammonium cations on the Cd sorption. The added Cd to soils was transformed from easily extractable fractions to more stable fractions, mainly residual, with increasing time of contact between the soils and cadmium. However, relatively high proportions of Cd were still present in the labile, exchangeable fraction of the soils after 180 days of the incubation study, indicating that Cd might pose an environmental risk to groundwater contamination in the area. The soils were highly efficient sorbents of Cd, when up to 99.8% of the total added Cd was sorbed during 60 min. The high sorption capacity of the soils for Cd was attributed to their alkaline character, high calcium carbonate, silt and clay contents. Addition of NH4NO3 to the aqueous phase had slight inhibitory effect on the sorption of Cd and the decreases in Cd sorption were in a range from ~0 to 3.3%. The results of the modified batch technique confirmed that the soil particles less than 10 μm sorbed a substantial proportion of Cd, and these particles might carry cadmium towards groundwater via soil macropores mainly during heavy rains, frequency and intensity of which are increasing as a result of climate change. The NH4NO3, HNO3, and EDTA solutions used as single extractants exhibited variable ability to extract Cd from the soils and the following order of their increasing extraction ability was observed: NH4NO3 < HNO3 < EDTA.
References
Abollino O, Aceto M, Malandrino M, Mentasti E, Sarzanini C, Petrella F (2002) Heavy metals in agricultural soils from Piedmont, Italy. Distribution, speciation and chemometric data treatment. Chemosphere 49:545–557
Bergkvist P, Jarvis N, Berggren D, Carlgren K (2003) Long-term effects of sewage sludge applications on soil properties, cadmium availability and distribution in arable soil. Agric Ecosyst Environ 97:167–179
Ingwersen J, Streck T (2006) Modeling the environmental fate of cadmium in a large wastewater irrigation area. J Environ Qual 35:1702–1714
Roels H, Lauwerys R, Buchet JP, Bernard A (1981) Environmental exposure to cadmium and renal function of aged women in three areas of Belgium. Environ Res 24:117–130
Hamzah A, Arifin WNWM, Khoo KS, Lee LJ, Sarmani SB (2009) Screening of biosorption bacteria tolerance towards copper and cadmium from oil sludge pond. J Radioanal Nucl Chem 281:295–298
de Matos AT, Fontes MPF, da Costa LM, Martinez MA (2001) Mobility of heavy metals as related to soil chemical and mineralogical characteristics of Brazilian soils. Environ Pollut 111:429–435
Adhikari T, Singh MV (2003) Sorption characteristics of lead and cadmium in some soils of India. Geoderma 114:81–92
Palágyi Š, Salzer P, Mitro A (2006) Sorption, desorption and extraction of cadmium from some arable and forest soils. J Radioanal Nucl Chem 269:103–113
Horckmans L, Swennen R, Deckers J (2007) Retention and release of Zn and Cd in spodic horizons as determined by pHstat analysis and single extractions. Sci Total Environ 376:86–99
Hooda PS, Alloway BJ (1998) Cadmium and lead sorption behaviour of selected English and Indian soils. Geoderma 84:121–134
Appel C, Ma LQ, Rhue RD, Reve W (2008) Sequential sorption of lead and cadmium in three tropical soils. Environ Pollut 155:132–140
Shaheen SM (2009) Sorption and lability of cadmium and lead in different soils from Egypt and Greece. Geoderma 153:61–68
Lichner L (1998) Cadmium transport in a loamy soil as influenced by macropore flow (in Slovak). J Hydrol Hydromech 46:207–217
Vogel T, Lichner L, Dusek J, Cipakova A (2007) Dual-continuum analysis of a cadmium tracer field experiment. J Contam Hydrol 92:50–65
Meers E, Du Laing G, Unamuno V, Ruttens A, Vangronsveld J, Tack FMG, Verloo MG (2007) Comparison of cadmium extractability from soils by commonly used single extraction protocols. Geoderma 141:247–259
Rao CRM, Sahuquillo A, Lopez Sanchez JF (2008) A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water Air Soil Pollut 189:291–333
Todorov B, Pekov G, Djingova R (2008) Fractionation of 137Cs and 60Co in soils by sequential extractions. J Radioanal Nucl Chem 278:9–15
Kabata-Pendias A (2001) Trace elements in soils and plants, 3rd edn. CRC Press LLC, Boca Raton
Fendorf S, Force MJL, Li G (2004) Temporal changes in soil partitioning and bioaccessibility of arsenic, chromium, and lead. J Environ Qual 33:2049–2055
Lu A, Zhang S, Shan X (2005) Time effect on the fractionation of heavy metals in soils. Geoderma 125:225–234
Jalali M, Khanlari ZV (2008) Effect of aging process on the fractionation of heavy metals in some calcareous soils of Iran. Geoderma 143:26–40
Kjoer C, Bruus PM, Elmegaard N (1998) Effects of soil copper on black bindweed (Fallopia convolvulus) in the laboratory and in the field. Arch Environ Contam Toxicol 35:14–19
Lock K, Janssen CR (2001) Ecotoxicity of zinc in spiked artificial soils versus contaminated field soils. Environ Sci Technol 35:4295–4300
McLaughlin MJ (2001) Ageing of metals in soils changes bioavailability. Environ Risk Assess 4:1–6
FAO (1998) World reference base for soil resources. World Soil Resources Report no. 84. Food and Agriculture Organization of the United Nations, Rome, Italy
Fulajtár E, Barančíková G, Čurlík J, Sedláková B, Šurina B (1998) Impact of the Gabčíkovo water work on agricultural soils (in Slovak). Soil Science and Conservation Research Institute, Bratislava
Nováková K, Nágel D (2009) The influence of irrigation on nitrates movement in soil and risk of subsoil contamination. Soil Water Res 4(special issue 2):S131–S136
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Takeda A, Tsukada H, Hisamatsu S, Inaba J, Takaku Y, Nanzyo M (2006) Extractability of major and trace elements from agricultural soils using chemical extraction methods: Application for phytoavailability assessment. Soil Sci Plant Nutr 52:406–417
Tipping E, Rieuwerts J, Pan G, Ashmore MR, Lofts S, Hill MTR, Farago ME, Thornton I (2003) The solid-solution partitioning of heavy metals (Cu, Zn, Cd, Pb) in upland soils of England and Wales. Environ Pollut 125:213–225
Berrow ML, Mitchell RL (1980) Location of trace elements in soil profiles: total and extractable contents of individual horizons. Trans R Soc Edinb 71:103–121
Ure AM (1996) Single extraction schemes for soil analysis and related applications. Sci Total Environ 178:3–10
Lichner Ľ, Čipáková A (2002) Cadmium distribution coefficients and Cd transport in structured soils. Rostl Výr 48:96–100
Lichner L, Dlapa P, Sir M, Cipakova A, Houskova B, Fasko P, Nagy V (2006) The fate of cadmium in field soils of the Danubian lowland. Soil Till Res 85:154–165
Mann SS, Ritchie GSP (1994) Changes in the forms of cadmium with time in some western Australian soils. Aust J Soil Res 32:241–250
Tang XY, Zhu YG, Cui YS, Duan J, Tang L (2006) The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China. Environ Int 32:682–689
Gommy C, Perdrix E, Galloo JC, Guillermo R (1998) Metal speciation in soil: extraction of exchangeable cations from a calcareous soil with a magnesium nitrate solution. Int J Environ Anal Chem 72:27–45
Christensen TH (1984) Cadmium soil sorption at low concentrations. I. Effect of time, cadmium load, pH and calcium. Water Air Soil Pollut 24:105–114
Palágyi Š, Rigas J (2004) Sorption of cadmium in some arable and forest soils. J Radioanal Nucl Chem 261:255–261
Alemayehu E, Lennartz B (2009) Virgin volcanic rocks: kinetics and equilibrium studies for the adsorption of cadmium from water. J Hazard Mater 169:395–401
Galamboš M, Kufčáková J, Rajec P (2009) Adsorption of cesium on domestic bentonites. J Radioanal Nucl Chem 281:485–492
Galamboš M, Kufčáková J, Rosskopfová O, Rajec P (2010) Adsorption of cesium and strontium on natrified bentonites. J Radioanal Nucl Chem 283:803–813
Ho YS, Wase DAJ, Forster CF (1996) Kinetic studies of competitive heavy metal adsorption by sphagnum moss peat. Environ Technol 17:71–77
Bagherifam S, Lakzian A, Ahmadi SJ, Rahimi MF, Halajnia A (2010) Uranium removal from aqueous solutions by wood powder and wheat straw. J Radioanal Nucl Chem 283:289–296
Dusek J, Vogel T, Lichner L, Cipakova A (2010) Short-term transport of cadmium during a heavy-rain event simulated by a dual-continuum approach. J Plant Nutr Soil Sci. doi:10.1002/jpln.200800281
Wang JJ, Harrell DL (2005) Effect of ammonium, potassium, and sodium cations and phosphate, nitrate, and chloride anions on zinc sorption and lability in selected acid and calcareous soils. Soil Sci Soc Am J 69:1036–1046
He ZL, Zhang M, Yang XE, Stoffella PJ (2006) Release behavior of copper and zinc from sandy soils. Soil Sci Soc Am J 70:1699–1707
Acknowledgements
This work was supported by the Scientific Grant Agency VEGA Projects No. 2/0170/09 and No. 1/0312/08.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Čipáková, A., Hiller, E. & Lichner, Ľ. Interaction and fractionation of added cadmium in some typical soils of the Danubian Lowland. J Radioanal Nucl Chem 287, 157–165 (2011). https://doi.org/10.1007/s10967-010-0667-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0667-5