Abstract
This study deals with an efficiency of a low dose of citric acid soil application on phytoextraction of uranium. Willow (Salix spp.) and sunflower (Helianthus annus L.) were tested in this experiment with contaminated soil. The enhancing of uranium bioaccumulation was confirmed, but in contrast to previous studies, the highest quantity of uranium was accumulated in leaves. After 5 weeks of citric acid treatment, willow was more efficient in the uptake and translocation of uranium than sunflower. The transfer coefficient calculated for leaves increased from 0.033 (control) to 0.74, or 0.56 after five doses of 5 mmol of citric acid per 1 kg of soil for willow or sunflower, respectively. The uptake characterized by the total U content achieved 88 and 108 mg kg−1 in relation to the above ground parts of sunflower and willow, respectively. Even though both plants accumulated U in their above ground parts in significant rate, they employed diverse ways to achieve it. At the end of the treatment, the physiological condition of the plants enabled us to continue this method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The history of the uranium industry in the Czech Republic comprises various intensities of mining and milling. Intensive activities were carried out from the 1950s to the 1990s. Nowadays the mining industry has declined but the five-decades of operation have resulted in a damaged environment. The worst impact belongs to chemical mining in the region called Ralsko [1]. Beside contamination of ground water during underground U leaching by sulfuric and nitric acid, sludge beds filled with the waste solutions of chemical mining have become a source of contamination to the immediate surroundings. The sources of uranium are small seepages and overflow of the open-air sludge beds by heavy rain. This soil served us for the experiment.
To decontaminate the land surface by an environmentally friendly technique, the phytoextraction method is being developed. Plants producing large biomass in a short time are chosen to determine the uptake and translocation of uranium. The sunflower represents a well recommended species for phytoextraction. It is proved to have a potential for U rhizofiltration as well [2]. Singh et al. [3] demonstrated on sunflowers a U distribution in the plant’s parts. Plants grown on soil spiked with U accumulated mainly in its roots and only a negligible portion was translocated to its leaves.
The willow is a common plant for studies of heavy metals phytoextraction. Its tolerance to a high dose of heavy metal is well known [4, 5]. Due to its suitable growth in less fertile conditions there is a possibility to test willow for uranium phytoextraction. As far as we know, no data has been published to date on uranium induced phytoextraction by willow.
Uranium has a small soil–plant transfer coefficient, typically, e.g., 0.0003–0.034 [6] or 0.005 [7]. Undoubtedly, uranium is transported to plants in natural conditions owing to imperfect selectivity by a plant’s uptake [8]. Whether this is the same mechanism for the uptake induced by citric acid still remains unanswered. Uranium is transported to plants mainly as uranyl (UO2)2+. Günther et al. [9] stressed the most probable bond created by the uranyl, namely uranyl phosphate to the phosphoryl groups. They uncovered this result with lupine plants (Lupinus angustifolius L.) and verified it on dandelions (Taraxacum officinale W.) and lamb’s lettuce (Vallerianella locusta L.). The only way plants may take up any compound from soil is to release it into a soil solution. Plants themselves have some mechanisms through their root exudation to enhance element concentration in the rhizosphere. The release of organic acids from the root has been suggested as a general mechanism for metal solubilization from the soil insoluble mineral phase [10]. Citric acid belongs to an organic complex agent produced by plants creating highly stable complexes with metals. This reaction is strongly pH dependent and is also influenced by solid phase sorption/desorption reactions, slower diffusion rates, microbial degradation and hydrolysation of organic acids by metal oxides [10]. Huang et al. [11] reported that U–citric acid complexes in calcareous soil seemed to be short lived due to rapid biodegradation at pH 8–9. Nevertheless, in acid soil, these complexes are more stable and U was apparently transferred to a shoot as U–citrate complex. The soil pH must decline below 5.0 to effectively transport uranium to the shoots [12]. In calcareous soil, there is a significant increase in soil solution and root U concentration, but little U is transferred to the shoots. Uranium probably accumulates on the surface of the roots or precipitates inside the roots in calcareous soil, whereas in acid soil, U accumulates in the shoots [13]. The cited article presented the results of the addition of 20 mmol of citric acid kg−1. The application of the organic agent increased the U concentration in the shoot by up to 1,400 mg U kg−1 or by 150-fold. At first, citric acid removes uranium into the soil solution by desorption caused by complexation or acidification of soil. Huang et al. [14] observed about a 200-fold increase of the U concentration in the soil solution after treatment by 20 mmol kg−1 of citric acid. Using the same concentration of citric acid, Shahandeh desorbed 78–82% of the total U content of acid soil. An advantage of citric acid compared to the synthetic complexing agent (EDTA) inheres in its low toxic effect on plants and biodegradation in soil in a rather short time.
Shahandeh and Hossner [13] reported that the main portion of U was accumulated in the roots, where its concentration can reach up to quantities 100-fold higher than in the shoots. For sufficient phytoremediation, it is more advantageous to reach translocation from underground to the above ground biomass of a plant. Moreover, they mentioned a direct relationship between the rate of soil contamination and the U accumulation in a plant.
Our investigation is focused on the soil–plant transfer of uranium induced by citric acid. This process is quantified using the soil–plant transfer factor (TF) defined as the ratio of the content of uranium in dry plants (mg/kg) to the content of uranium in dry soil mg/kg (Eq. 2). Given more definitions of TF, it can acquire enough different values. Moreover, TF is influenced by a number of factors, e.g., pH and other soil specific properties, speciation of uranium in soil, content of organic matter, etc. The TF must be related to a particular part of a plant or to a whole plant due to different accumulating features of uranium.
Apart from testing willow for its phytoextraction potential, the primary aim of this paper is to reach significant uranium uptake by the above ground biomass after an application of low doses of citric acid into soil.
Materials and methods
Experimental soil
Soil originated from the area surrounding an old chemical mining facility, where acid extraction had been done. For the experiment we used forest soil contaminated by a seepage or overflow of the sludge bed. The content of U in soil determined by High Purity Germanium (HPGe) gamma spectrometry was 480 ± 40 mg kg−1. The soil characteristics are summarized in Table 1. It was characterized as a cambisol.
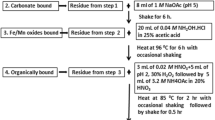
To obtain more detailed information of uranium bindings in soil we accomplished sequential analyses based on Tessier’s method [15]. The exchangeable uranium was extracted by 1 M MgCl2 at pH 7.0 in the first step. The consecutive extraction by 1 M CH3COONa at pH 4.8 modified by CH3COOH was designated for the leaching of an aluminium–silicate fraction. The next step proceeded to the extraction by 1 M NH2OH.HCl in 25% acetic acid during a 14 h period. To release the following fraction of uranium, 5 mL of 0.02 M HNO3 was used and 15 mL of 30% H2O2 at pH 2 and 85 °C over 2 h. After adding the next 3 mL of 30% H2O2 the leaching resumed. In the original method it was designated for the extraction of an organic fraction. Uranium leached in this step may also originate from its bond to sulphur. Further tetravalent uranium is included in this fraction. The remaining uranium was determined after digestion of the residual by HNO3 (8 mL), HCl (3 mL) and HF (2 mL) in the microwave oven at 210 °C. The leach of the first and second step was preserved using 1 M HNO3. The results of the procedure are summarized in Fig. 1.
Content of uranium in the fractions of soil determined by sequential analyses. Extraction agents for the particular fractions: 1—MgCl2; 2—CH3COONa; 3—NH2OH.HCl; 4—H2O2/HNO3; 5—residual. The control variant represents the samples of soil without citric acid treatment. Interval of measurement uncertainty has a level of confidence of approximately 95% (k = 2)
To determine the total content of uranium by ICP-OES, the 0.5 g of the soil sample was digested by HNO3 (8 mL), HCl (3 mL), HF (2 mL) in the microwave oven at 210 °C. Furthermore, the acid was evaporated at 140 °C in Teflon vessels. Finally, evaporated salts were digested by aqua regia. Of the analysis, the U content in soil was shown to be 520 ± 40 mg kg−1.
Pot experiment
The plant’s experiment took place in an open-air greenhouse. The willows and sunflowers were planted for 1 and 2 months, respectively in pots with a 30 cm diameter and 5 kg content of soil. To enhance the soil fertility, we amended 0.1 g kg−1 (N), 0.04 g kg−1 (P), 0.1 g kg−1 (K) to soil for the willows (Salix smithiana Willd.) and 0.2 g kg−1 (N), 0.07 g kg−1 (P), 0.2 g kg−1 (K) for sunflower (Helianthus annus Alexandra) in a form of ammonium nitrate and potassium phosphate. In both cases, we designed three treatments differing in the number of citric acid applications. Four sunflower seeds were inserted into the soil of each pot. One willow cutting was planted into each pot. In the first treatment (control), no citric acid was applied. After 1 month of growth, the treatment with the 0.08 mol.l−1 solution of citric acid began. A dose of 300 mL of the solution was poured over the top of the pots once in the second treatment and five times in 5 weeks in the third treatment. In the second treatment, one dose of citric acid was applied: the total amount of citric acid was 5 mmol per 1 kg of soil. In the third treatment, five doses of agent were applied during a 5 week period: the total amount of citric acid was 25 mmol per 1 kg of soil. All treatments were replicated three times.
Plant analysis
One week after the last dose of citric acid, we harvested all of the plants to make the analyses. The flowers, leaves, stems and roots of the plants were separated, washed by purified water (<4.3 μS/cm) and air dried at 40 °C. To analyze by ICP-OES, the plant’s samples were dissolved in 7 mL of HNO3 and 2 mL of H2O2. The process was accelerated with the aid of a microwave oven Ethos 1 (MLS GmBH, Germany) at 180 °C max. and calibrated by the uranium ICP standard (ULTRA Scientific, ICP-092).
U mobilization was tested in the extraction with 20 mmol kg−1 of citric acid. U deposited in a 10 g of sieved soil was extracted by 50 mL of citric acid during 1 h of intensive shaking. The U yield was 57 ± 0.7 mg from 1 kg of soil or 21% of the uranium content in the soil determined by ICP-OES.
We analyzed the samples by High Purity Germanium (HPGe) gamma rays spectrometry (Canberra, model GC 1018-7500SL, relative efficiency 10%) and inductively coupled plasma optical emission spectrometry (ICP-OES, Vista Pro, Varian, Australia).
Non-destructive analyses by HPGe detector determines isotope 235U by the calibration curve using the software Gennie 2000. The activity of 235U is assessed from 143.8, 163.4 and 205.3 keV spectral lines. The leaves were compressed into 100 mL vessels. The activities were corrected using coefficients due to a difference in density between the calibration standard and the samples. By virtue of wide spectral analyses, we can also determine another nuclide with gamma radioactivity, e.g., 40K.
Statistical evaluation
Results of the U content in plant tissues were presented as an arithmetic mean with uncertainty having a level of confidence of approximately 65% (k = 1). Each plant sample was analyzed twice.
The uncertainties of the results gained, with the help of gamma spectrometry, were presented with a combined standard uncertainty. The measurement took around 60,000 s. The combined standard uncertainty takes into consideration the uncertainty of measurement (evaluated by the software Genie 2000), putting the sample on the detector, a sample’s homogeneity and uncertainty of half-life of radionuclide, etc. Its conservative estimate is approximately 10%.
Results and discussion
Bioavailable fraction of uranium
Changes observed in the uranium content in the soil fractions displayed in Fig. 1 imply a significant decline in the content of uranium leaching using CH3COONa/CH3COOH. With regards to the origin of soil, this fraction is associated with aluminium–silicate and uranium is released from it by the same agent as in the case of carbonate fraction. The decrease of U deposited in the organic matter is within the frame of standard deviation, therefore insignificant. One of the ways uranium could be located in organic matter is through its return to the soil after plant tissue decomposition [8]. Newly created conditions after citric acid treatment caused a small amount of growth of the U sorption on Fe/Mn oxides. The result suggests that the greatest portion of uranium in the soil solution originated from the second fraction.
For U uptake the P deficiency in soil can play an important role. The original P content was 40 mg kg−1. Nutrients were supplied to the soil in regular amounts at the beginning so that the plants would not experience deficiency. In the pH range of 4.5–9, phosphor coupled with uranyl forms stable complexes [12]. As a result the concentration free uranyl in soil solution declines and U becomes hardly available for plants.
A lower P content, uranium binding to the extractable fractions, a lower content of organic matter, and an acid pH of soil are all parameters supporting the U uptake by plants.
Accumulation and distribution of U in plant
Many previous studies [13, 16] referred to accumulation in the above ground parts of a plant as less than in that of the roots. They explained the dominant accumulation on the basis of precipitation or sorption of uranium in roots [13]. Our results partially contradict the cited literature. The accumulation in the leaves is significantly higher than in the roots, and this can be caused by low doses of the complex agent.
The efficiency of the citric acid treatment is evident in Fig. 2. Uranium in the complex with citric acid was transported by the transpiration flow to the leaves. The accumulation of uranium in the willow leaves in the third series rose to a value 22–26 higher than in the control series. The sunflower leaves showed an increase of 18-fold. The U concentration in the leaves of the control treatment reached the same values (16 ± 5 mg kg−1) as in the case of sunflower shoots presented in Shahandeh and Hossner [13] or Singh et al. [3]. There was no difference in the mean weight of the sunflower leaves when compared to the series. The accumulation in the leaves is a practical advantage. The above ground part is easily harvested and in the case of willow, the tree can grow several years regardless of the treatment because the small doses of citric acid allow it to endure the long-time application.
After the separation of the parts of the sunflower’s body and their analysis, we noted a similar distribution of U across the treatment variants. The bioaccumulation of uranium occurred in the roots, stems, leaves and flowers of the sunflower as 15–30%, 2.0–3.0%, 65–79% and 2.2–3.9%, respectively. In the control variant, uranium was distributed in the plant’s body, subsequently: 65–71% in the roots, 4.8–12% in the stems, 15–18% in the leaves and 6.3–7.8% in the flowers. We calculated this value using Eq. 1. To correctly assess the distribution in the plant’s body we must take into consideration their individual weight.
where w U is the content of uranium in a plant’s part as a portion of the total uranium content in a plant, c x is the content of uranium (mg per 1 kg of dry weight of plant) in a part of a plant, m x is the weight of a plant’s part, c is the total content of uranium in a plant per 1 kg of a dry mass, m is the dry weight of a plant.
Willow showed different distribution of uranium during the treatment by citric acid (Table 2) than sunflower plants described above. In the control variant the highest portion of U was localized in the stem (69%; Table 2). As the doses were applied the mutual proportion of U almost equalized. Finally the major portion of U built up in the roots and leaves. Indeed, we shall take into consideration that during the growth the proportion between parts of plant’s body has been changed.
In the sunflower, a dominant portion of U was located either in the roots (control variant) or in the leaves (treated variants) as listed in Table 3. This means that the transport from a root to a shoot significantly increased. In the treatment variants uranium is not precipitated in the root at such a large extent than in the control, but rather transported in any mobile species to the leaves. In other words, citric acid mobilizated just this portion of U accumulated in a root zone.
Comparison of U contents in the roots of willow and sunflower shows the higher natural accumulation in the sunflower’s roots.
Table 3 demonstrates that uranium is significantly less concentrated in flowers than in leaves and roots. From this perspective, uranium behaves similar to Zn and Cd which are also preferentially translocated into leaves [17].
Transfer factor was calculated by the Eq. 2.
where C p is the content of U in dry mass of plant or (plant’s part) at the end of experiment and C s is content of U in dry mass of soil at the start of experiment
The TF of uranium regarding the leaves was only 0.033 for control variants, 0.11 for willow and 0.20 for sunflower after one treatment, 0.71 for willow and 0.56 for sunflower after five treatments. The results are summarized in Table 3. If we compare the results with, e.g., Singh et al. [3] who carried out the experiments on spiked soil (400 mg of U per 1 kg of soil), we observe a surprising likeness (TFroot = 0.58; TFstem = 0.070; TFleaves = 0.042). In contrast to their experiment we planted sunflowers on soil with long term stabile contamination.
The effect of toxicity on the shoots was noted on the leaves, mainly in the lower parts of the stems. However, the physiological conditions of the plants were not damaged by the treatment to such an extent that required aborting of the experiment. The plant recovered from the toxicity and we were able to observe flowers of the sunflowers and foliation on the lateral branches of the willows.
The total U content, calculated by Eq. 3, in the control variant of the sunflower was 44 mg (U) per 1 kg of dry mass and 14 mg kg−1 was transported to the above ground part. Within the second treatment it achieved 38 mg (U) kg−1 of dry mass and 34 mg kg−1 was transported to the above ground part. As far as the third treatment is concerned, the total uptake of U was 99 and 88 mg kg−1 was transported to the above ground part. This implied that U accumulation in the above ground part of sunflower increased by a factor of 6 between the control and third treatment. Similarly the U content in willow is presented in Table 4.
where c is the total content of uranium in a plant per 1 kg of dry mass, c x is the content of uranium (mg per 1 kg of dry weight of plant) in a part “i” of a plant, and w x is the mass fraction of a i-part to all of a plant
We also received encouraging results by virtue of a small portion of U absorbed by ferric oxyhydroxides and organic materials which can reduce bioavailability of U6+ [8]. We achieved a significant increase of U transport to the plant’s leaves after five doses of citric acid comparable to the increase after an application of one high dose presented in [13].
Uptake potassium, iron and zinc attached to uranium induced phytoextraction
We also obtained information about the transport of some other elements (Table 1). In accordance with the results of Jones and Darrah [10], only a small effect of citric acid on potassium was observed, likely as a consequence of the acidification. Vanhout et al. [18] observed a decrease of potassium in leaves during uranium treatment affecting membrane integrity and functionality. As well as Mordechai et al. [19] said about a decrease of potassium in roots accumulating uranium. On the basis of the non-decreasing potassium content we can suppose a good physiologic condition of the experimental plants. Uranium has a lower toxic effect on cells attached to the uptake induced by low doses of citric acid than in the previous experiments.
Nascimento et al. [20] described the enhancing of bioavailability of zinc due to complexing with citric acid. Furthermore, Utmazian and Wenzel [21] observed the high Zn accumulation in leaves employing Smithiana clone of willow. More surprising than the high Zn content is its decrease attached to the number of doses of citric acid. The competition with other ions in complexation with citric acid played most likely the important role in decrease of the Zn content. However, it is evident from Table 5, that uranium together with Zn, affected a plants health.
Iron makes a complex with citric acid quite easy [10, 11] what can decline the uptake of U complex. The Fe content in leaves enhanced with the number of citric acid doses.
Many other elements, e.g., K, Pb, Cu, Cd, As, Ni did not accomplish a decisive increase in their leaf concentrations and, with the exception of K, their content was small in the leaf tissues.
Comparison willow and sunflower in uranium uptake
The underlying differences between the species of plants that were used are:
-
The U accumulation in the root of sunflower already occurring in the control variant.
-
The difference in distribution of uranium in a plant’s parts relates to the number of citric acid doses.
The accumulation of U in the roots of the sunflower confirmed the predisposition of this species for rhizofiltration use. U moving up from the roots to the above ground parts, after the treatment by citric acid, resulted in the decrease of the total content of U in the roots and its increase in the leaves.
Willow during the treatments by citric acid had steadily accumulated uranium in its roots and leaves.
The difference between accumulation of U in the sunflower and willow after the first and the fifth treatment can be caused by a different growth period. Soon after germination, the sunflower produced a high quantum of biomass in a short time. The willow was planted from twigs without young shoots and leaves. The rate of the biomass production may be the cause of this phenomenon.
The transfer coefficient in the control treatment is slightly higher than in natural conditions. This is to be expected due to higher concentrations of uranium in the soil.
Conclusion
Despite the published papers, we split the application into small doses of a complex agent instead of one high dose. The plants have a better resistance to the toxic effect of the acid and uranium. The uranium transport from the roots to the shoots is made more effective by using a low dose of citric acid. The willow manifested itself as a perspective mean for phytoremediation of soil contaminated by uranium. This management can be enforced on soil where food production is facing threat. It would require a collection of annual shoots and leaves of mature trees periodically treated by low doses of citric acid.
References
Mudd GM (2001) Critical review of acid in situ leach uranium mining: 2. Soviet Block, Asia. Environ Geol 41(3–4):404–416
Dushenkov V et al (1995) Rhizofiltration—the use of plants to remove heavy metals from aqueous streams. Environ Sci Technol 29, 1, s.:1239–1245
Singh S et al (2005) Uranium uptake studies in some plants. Radiat Meas 40:666–669
Unterbrunner R et al (2006) Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environ Pollut 148, is. 1: 107–114
Vyslouzilova M et al (2006) Rhizosphere characteristics, heavy metal accumulation and growth performance of two willow (Salix × rubens) clones. Plant Soil Environ 52:353–361
Vandenhove H, et al (2007) Can we predict uranium bioavailability based on soil parameters? Part 2: Soil solution uranium concentration is not a good bioavailability index. Environ Pollut 145, is. 2: 577–586
Hinton TG et al (2005) Phytoextraction of uranium and thorium by native trees in a contaminated wetland. J Radioanal Nucl Chem 264(2):417–422
Morton LS et al (2001) Pedogenic fractionation and bioavailability of uranium and thorium in naturally radioactive spodosols. Soil Sci Soc Am J 65:1197–2003
Günther A et al (2003) Uranium speciation in plants. Radiochim Acta 91:319–328
Jones LD, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Huang FYC et al (1998) Biodegradation of uranium–citrate complexes: implication for extraction of uranium from soil. Environ Sci Technol 32, 1, s.:379–382
Ebbs SD et al (1998) Role of uranium speciation in the uptake and translocation of uranium by plants. J Exp Bot 49(324):1183–1190
Shahandeh H, Hossner LR (2002) Enhancement of uranium phytoaccumulation from contaminated soils. Soil Sci 167(4):269–280
Huang JW et al (1998) Phytoremediation of uranium-contaminated soil: role of organic acid in triggering uranium hyperaccumulation in plants. Environ Sci Technol 32:2004–2008
Noseck U, et al (2008) Identification of uranium enrichment scenarios by multi-method characterisation of immobile uranium phases. Phys Chem Earth A/B/C 33, is. 14–16: 969–977
Laroche L et al (2005) Root uptake of uranium by a higher plant model (Phaseolus vulgaris)—bioavailability from soil solution. Radioprotection 40:33–39
Sauerbeck DR (1991) Plant, element and soil properties governing uptake and availability of heavy metals derived from sewage sludge. Water Air Soil Pollut 57–58(1):227–237
Vanhoudt N et al (2008) Effects of uranium and phosphate concentrations on oxidative stress related responses induced in Arabidopsis thaliana. Plant Physiol Biochem 46:987–996
Mordechai S et al (1988) Localization and toxic effects of cadmium, copper, and uranium in Azolla. Plant Physiol 88:30–36
do Nascimento CWA, Amarasiriwardena D, Xing B (2006) Comparison of natural organic acids and synthetic chelates at enhancing phytoextraction of metals from a multi-metal contaminated soil. Environ Pollut 140:114–123
Utmazian NDS, Wenzel WW (2007) Cadmium and zinc accumulation in willow and poplar species grown on polluted soils. J Plant Nutr Soil Sci 170, is. 2, s.: 265–272
Mehlich, A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Soil Sci Plant Anal 15, is. 12: 1409–1416
Acknowledgments
We are grateful to the Czech University of Life Science for its support and for the use of their analytical instruments. We would also like to thank the National Radiation Protection Institute for the use of their analytical instruments. This project was financed by Czech Science Foundation GACR 104/07/0977 and by the research program of the Ministry of Education, Youth and Sport project No. MSM 6046070901.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mihalík, J., Tlustoš, P. & Szaková, J. Comparison of willow and sunflower for uranium phytoextraction induced by citric acid. J Radioanal Nucl Chem 285, 279–285 (2010). https://doi.org/10.1007/s10967-010-0538-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0538-0