Abstract
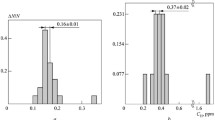
The hydrogen concentration for the reference material low-alloy steel was estimated using prompt gamma neutron activation analysis. The hydrogen concentration had a range of 0.91–1.14 mg/kg as measured by the prompt gamma activation method but the reference value was about 1.0 mg/kg as measured by the ICP/AES method. The relative error appeared to be 14% and the differences showed as the background by blank. The standard reference material (NIST SRM) was used for the analytical control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A nondestructive on-line measurement for metal samples was analyzed using prompt gamma neutron activation analysis. Because the neutrons and gamma rays were highly penetrating for most materials, this analytical method was performed non-destructively and in situ, removing all questions of incomplete dissolution, contamination and sample loss. During measurement under neutron irradiation, the hydrogen background gamma rays appeared by the capture of scattered neutrons from the sample and sample holder, and a careful measurement and analysis of the background thus became necessary. According to the Refs. [1, 2], the contribution of the background prompt gamma rays to a sample spectrum was known to vary depending on the composition of the matrix under irradiation. In the case of such a background where the gamma rays interfere with the gamma rays of the analyte, a background correction becomes crucial. When the sample is irradiated together with a vial, it is necessary to measure a blank. Such measurement allows for correction of the background generated from the neutron capture by the environmental hydrogen and also for the background due to spectral interference.
The samples used were of the granule type that was irradiated while attached to a paper filter. Generally, it is somewhat difficult to measure hydrogen because the samples are easily influenced by moisture in the sample as well as environmental and background effects. Because powder-type samples are highly sensitive under hydrogen analysis for the above reasons, a granule-type sample on metal is more useful than powder. But these samples also must have their moisture degree checked as soon as the sample is prepared and dried. The interference peaks by the measurement condition of the sample position as a system matrix and the background level in the hydrogen energy region as a sample matrix were investigated periodically.
In the present study, a few samples of low-alloy steel were used as a reference material (RM) to obtain the hydrogen content and the standard reference materials (SRM) were also irradiated for analysis assurance. For elemental analysis of low-alloy steel that was used for development as certification-standard materials, the RM and SRM samples were irradiated with neutrons under equal conditions. In this research, the RM and SRM (NIST 364) for the low-alloy steel were made in the form of identical disks (13 mm) to minimize the geometric uncertainty that occurs during neutron irradiation and gamma-rays measurement.
Experimental
The present study is based on measurements using the same facility used reference [3]. The low-alloy steel material was made using the mold. The mold used to make the standard sample had an upper part measuring 410 × 410 mm and a lower part measuring 300 × 300 mm that was in the shape of an inverse lozenge. The procedures for preparing reference materials are as follows: each ingredient was introduced into the 1,000 kg vacuum induction furnace for melting. This was followed by, casting of an ingot and cutting of the top and bottom with a band saw. The ingot was then forged after reheating at 1050 °C for 1 h, followed by homogenizing treatment at 900 °C for 4 h, billet rolling and repeat homogenizing. Finally, it was cut to the appropriate size to provide a reference material that could be machined to chip, bar or disc form and then stored. The designed chemical compositions for the reference materials of low-alloy steel were mainly fluorine of 97.61–97.66 m/m%, and about 1.5 m/m% among the remainders were carbon, manganese, phosphorous, silicon and sulfur as the main element and other elements were below 1 m/m%.
The low-alloy steel samples used were attached with one layer on the compressed paper sheet and had a diameter of 8 mm. A granule of the sample had a size of 0.5 mm, and was shaped like rhombus with intervals of 3 mm on a paper sheet to reduce neutron self-shielding, scattering and gamma-ray attenuation. The standard reference material was also prepared with the same method. The sample granule within the paper sheet was wrapped in a thin Teflon sheet to prevent dropping. The blank made with paper, which was wrapped with a thin Teflon sheet, was irradiated to confirm the background and interference peaks appearing from the paper. The RM and SRM of the low-alloy steel of about 100 mg weights were each measured with 10,000 s.
Measurement and calculation
Neutrons captured by hydrogen in the shielding materials and in the atmosphere gave rise to hydrogen background gamma rays in the prompt gamma neutron activation facilities. The magnitude of this background was dependent upon the matrix of the sample and surrounding components. For this effect, the background counts of hydrogen gamma-rays were evaluated from irradiation of a blank. To confirm the background effect for determining the concentration of hydrogen, it is necessary to measure the blank spectrum prior to sample measurement. The count rate of the hydrogen peak arising from the off-neutron beam was estimated to be about 0.07 ± 0.01 cps corresponding to approximately 13 μg of hydrogen. The count rate for the interference peaks without a blank appeared to be about 0.20 ± 0.01 cps in the measurement for 10,000 s.
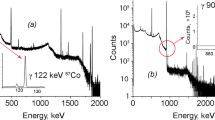
As shown in Fig. 1, interference for the hydrogen peak region appeared with a few peaks. The background in the hydrogen gamma-energy region (2220–2225 keV) that was generated by means of a prompt gamma-ray spectrum measured for a hydrogen-free material arose from a few possible sources. The interference peaks were emitted by other elements in the sample matrix representing a possible secondary source of the background, which was distributed within 5 keV of the hydrogen peak energy. The background energies were 2221, 2223.3, 2224.4, and 2226.8 keV, where the remainders of the peaks except for 2222 and 2225 keV had an extremely small peak area. The total count rate in the hydrogen gamma energy region for the blank was about 0.43 cps and the percentage of the count rate per energy for the above four energies was 6, 21, 45 and 28%, respectively. Neutron capture by hydrogen in the atmosphere and shielding materials gave rise to about 0.2 counts per second of a gamma radiation. Generally, the count rate of the long-time measurement was lower than that of the short measurement. The count rate for the blank in the hydrogen energy region was about two times higher than that for the air condition of the on-beam stage. Before the sample was measured, the fluctuation of the count rate for the hydrogen peak was checked using a blank and the variations in the peak area appeared to be below 1% for 10,000 s measurement.
Results and discussion
The temperature of the measuring system was 24–25 °C at the sample position. The moisture and composition of the samples were continuously controlled by the sample device circumstances of the system. Also, the neutron flux monitoring system was checked using a blank and Ti foil before and after measurement of the sample for analytical control. The sensitivity of the analysis was dependent upon irradiation parameters such as neutron flux, irradiation and decay time, measurement conditions such as measurement time and detector resolution/efficiency, nuclear parameters of the elements such as isotope abundance, neutron cross-section, half-life and gamma-ray abundance, and the geometry of the distance from the source to the detector and the surrounding medium.
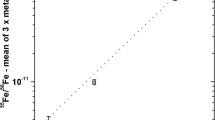
The sensitivity of hydrogen in liquid and powder matrices was carried out to determine the magnitude of the enhancement as a function of the hydrogen concentration. The calibration curves obtained from the liquid and powder of the standard reference materials are shown in Fig. 2. The results for chemicals such as explosive material including nitrogen and carbon showed a high statistical error and the fluctuations in the count rate were also high relative to long live-time measurement. The correction for the hydrogen concentration in the bio- and metal samples was performed using a calibration curve. The calibration curve was obtained from using the standard materials of mannitol, polyethylene, explosives of chemical compounds and melamine. The correlation coefficient was 0.1127 + 3.1078X. In the high mass fraction above 20 mg, the slope deviation between the two functions of the top and bottom line was about 5%, which gave rise to fluctuations of the standard deviation for measured concentration. The sensitivity and the detection limit of hydrogen were 3 cps/mg and 6 μg, respectively.
The CRMs of titanium-based alloy (173c) and hydrogen in titanium alloy (2453) were analyzed in the present work. Samples of weighing approximately 3–50 mg were placed in a 0.5 mm thick Teflon cylinder with an inner diameter of 6 mm and a height of 1 cm. The count rate for the blank in the hydrogen energy region was about 1.5 times higher than the environmental conditions of the on-beam stage. The hydrogen concentration of the SRM sample was determined from the measured count rate of the hydrogen peak, sample mass and hydrogen sensitivity. The results are summarized in Table 1 together with the certified values. The relative errors of the measured values are about 3.5% for a high hydrogen concentration such as SRM 2453, or about 6% for the 60 mg/kg concentration such as the titanium-based alloy. The analysis of the hydrogen content agreed well with the certified value of the NIST SRM.
The low-alloy steel, which was made from high-purity electrolytic iron with a purity of above 99.9% as the standard material for the boron analysis was used for the analysis using a calibration curve, as shown in Table 2. In determining the hydrogen, the content deviation appeared to be about 3% for the high mass fraction and high count rate but the results showed a slightly higher value for the calibration line obtained from the single chemical compound than for the line of the many compounds. The results for the same mass fraction and low content were almost the same for both single and multi compounds. In the case of each RM sample, the content of the hydrogen had the same results. The declination of the single chemical compound using the low sample weight appeared with a 5.4% difference for the high mass fraction region above 20 mg of the multi chemicals, but the obtained value was in good agreement with the calibration line of the multi chemicals. Therefore, the calibration function evaluated from those chemicals has proven useful in analyzing the hydrogen.
The spectrum for the low-alloy steel was shown in Fig. 3. The ratio of the hydrogen count in the low-alloy steel was not high, but the measured data appeared to be high because the blank used was compressed with a few paper filters that adhered to the sample. In the case of the hydrogen peak of the alloy steel sample, the interference peaks appear weakly with a few peaks. The sample below 0.5 mg was influenced the interference peaks that caused a fluctuation of more 8% in the hydrogen net peak. In the high mass above several mg, the net area by the interference peaks was small and the fluctuation of the net peak area did not show as much influence.
The reference value of hydrogen for an NIST SRM 364 sample was about 5 mg/kg, but the measured value was slightly higher than the estimated value. The measured values for the steel alloy materials showed a range of about 1 mg/kg, but the hydrogen concentrations of these samples have not yet been definitively reported using other methods. The combined uncertainty was about 3.4% for the hydrogen analysis. The statistical error, which included uncertainties arising from the observed radiation emitted in nuclear decay and which showed some degree of statistical fluctuation, was 2.2%. The second uncertainty associated with the background subtraction that gives rise to neutron capture by hydrogen in shielding and in the atmosphere, whose value was 2.3%. The sources of the other uncertainties were mainly the neutron flux variations for 10,000 s measurement (0.5%) and the spectral interference for low sample weight (1.2%), as shown in Table 3.
Conclusion
In this study, calibration curves were obtained from a few kinds of standard materials in liquid and powder form. To analyze low hydrogen concentrations below 1 mg/kg, the standard material of a liquid type has to be well enveloped to avoid loss. In measuring the powder sample, the sample weighing below 1 mg showed the strong influence of environmental conditions within the sample position, leading to high statistical error in the hydrogen energy region. When a low mass weight below 20 mg was used, the calibration line obtained from melamine gave at lower deviation than those of multi chemicals. Also, in the sample was used a high weight of above 20 mg, and the deviation width of content appeared to be within 5% from the calibration curve of the multi compounds. The analysis functions obtained by the different sample group showed a difference of a few percentage points, which ultimately influenced the uncertainty of the data analysis. The difference in content from each calibration was about 3%. In the case of the powder-type biological sample, the interference peaks appearing on the tail region of the hydrogen peak area was hidden but the background count rate was high because of the moisture effect.
The metal sample, which was not influenced by moistures, received the effects of strong interference peaks, which appeared as the count deviation from the background subtraction. The measured calibration curves were in good agreement with each other in term of declination but showed a deviation of a few percentage points for the metal; this deviation was included with uncertainty on the analysis of the real sample. The measurement results of the low-alloy steel for hydrogen content could be confirmed within a standard deviation for five reference materials, and the total average value was also close to estimated by other independent analysis methods (ICP/AES).
References
Paul RL (1997) Analyst 122:35R–41R
Paul RL, Lindstrom RM (2000) J Radioanal Nucl Chem 243:181–189
Cho HJ, Chung YS, Kim YJ (2005) Nucl Instr Method B 229:499–507
Lindstrom RM, Paul RL, Vincent DH, Greenberg RR (1994) J Radioanal Nucl Chem 180:271
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, HJ., Park, KW. Analysis of hydrogen concentration in low-alloy steel. J Radioanal Nucl Chem 284, 533–537 (2010). https://doi.org/10.1007/s10967-010-0506-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0506-8