Abstract
This work focuses on the “3 + 1” mixed ligands of 99mTc labeled Gabapentin as α2δ receptor imaging agents in the brain. Gabapentin 1-(aminomethyl)cyclohexanacetic acid as monodentate and two tridentates: tridentate A; 3-(2-imino-thiozolidin-4-one)-quinozoline-4-(3H)-one and tridentate B; N-(4-chlorophenyl)-2-imino-2H-chromene-3-Carbothioamide which were synthesized and characterized by infrared analysis (IR), 1H nuclear magnetic resonance (NMR), and mass spectrum. 99mTc-complexes were prepared by the “3 + 1” mixed ligand approach. The labeling conditions were optimized and the complexes was extracted by chloroform and purified by high performance liquid chromatography. 99mTc-complexs were lipophilic and stable for at least 8–12 h at room temperature. The biodistribution of the 99mTc-complexes was evaluated in mice. The brain uptake was 4.5% and 3.5% ID/g (percentage of the injected dose per gram) at 5 min, and the retention was 1.5% and 1.7% ID/g at 120 min for 99mTc-complex A and 99mTc-complex B, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The transport and accumulation into the target organ is an important issue for 99mTc radiopharmaceuticals development. The development of radiopharmaceuticals designed to bind specific receptors, including membrane transport systems, is receiving much interest due to their potential to achieve improved in vivo monitoring of biochemical and physiological functions [1]. Technetium-99m (99mTc) is the radionuclide of choice for diagnostic imaging with single photon emission computed tomography (SPECT) due to its ideal nuclear properties (Eγ = 140 keV, T 1/2 = 6 h, no β-emission) and availability from a 99Mo/99mTc generator [1]. These properties have led to the search of novel 99mTc-based radiopharmaceuticals incorporating ligands specifically designed to probe protein receptors and transporters [2–4]. A new 3 + 1 mixed-ligand approach has been proposed as a new strategy for developing novel neutral 99mTc-radiopharmaceuticals containing the Tc = O core [5, 6]. This approach, which consists of a tridentate ligand and a monodentate co-ligand surrounding the Tc = O core, has evolved successfully to generate novel lipophilic potential radiopharmaceuticals with high brain uptake and retention [7–9]. This strategy also offers an easy access to 99mTc-based probes with affinity and selectivity to protein receptors, in which the receptor ligand is appended either into the tridentate or the monodentate ligand [10–12].
One of the reported receptors ligands is WAY 100635, a potent antagonist of pre- and post-synaptic 5-HT1A receptors, with residue 1-(2-methoxyphenyl) piperazine [13]. Fragment of WAY 100635 was combined with different technetium tetradentate N2S2 chelates, amine-amide dithiols or diamine dithiols [14–17]. The major disadvantage of these compounds is their poor brain uptake in experimental animals which precludes their usefulness as brain receptor imaging agents. This has been attributed mainly to their high molecular size. However, “3 + 1” oxotechnetium mixed ligands complexes of the general type 99mTcO[SN(R)S][S] have been synthesized as 5-HT1A radioligands by introducing the receptor-binding 1-(2-methoxyphenyl)piperazine moiety on the monodentate ligands [18, 19]. These complexes showed in vitro affinity for 5-HT1A in nanomolar range and moderate brain uptake in mice and rats.
Also, Gabapentin (Neurontin®) 1(aminomethyl) cyclohexanacetic acid is anticonvulsant analgesic drug synthesized nearly 40 years ago [20]. The original concept was to increase the lipophilicity of the inhibitory neurotransmitter GABA (γ-aminobutyric acid) by addition of a cyclohexyl substituent, thereby increasing its CNS penetrating properties yet retaining a similar pharmacology. Recently, the [3H] Gabapentin binding protein was subsequently purified from pig brain and shown to be the α2δ subunit receptor of the voltage dependent calcium channel complex [21]. So the success in synthesizing “3 + 1” oxotechnetium mixed ligands complexes of Gabapentin (Fig. 1) will provide potential radiopharmaceuticals.
The present work is concerning the technetium-99m labeling of Gabapentin by the “3 + 1” mixed-ligand approach in which it acts as monodentate (L1) and using each of quinazoline derivative and thioamide derivative as tridentate (L2). Quinazolines are classes of fused heterocycles that are considerable interest because of their safe biological properties [22]. Also, thioamide derivatives are used for therapeutic purposes such as treatment of tuberculosis, leprosy, and thyrotoxicosis [23, 24]. Theoretically possible complexes produced from this labeling reaction are neutral mixed ligand complex (99mTc(O)L1L2), binuclear complex of the tridentate ligand [(99mTc(O))2(L2)3)] and anionic complex of the monodentate ligand [(99mTc(O)(L1)4]. Of all these complexes, the neutral mixed ligand complex is the most produced complex specially when the molar ratio of mono- to tridentate is 1:1 [25]. The presumable structure of 99mTc-complex A and 99mTc-complex B are shown in Figs. 2 and 3, respectively.
Experimental
Materials and methods
Gabapentin, 1(aminomethyl)cyclohexanacetic acid, was obtained as a gift from AMOUN PHARMACEUTICAL CO. Cairo, Egypt. (C9H17NO2), MW = 171.24.
Pertechnetate-99m solution was obtained by elution from the sterile 99Mo/99mTc generator (Elutic, Brussels, Belgium).
White Albino mice were used for biodistribution studies.
Synthesis and structure confirmation of tridentate ligands
Synthesis of 2-imino-thiazolidin-4-one derivative of 4(3H) quinazoline (A)
Addition of benzoxazine derivative [1] to hydrazine hydrate gives 3-amino-quinazoline-4(3H)-one derivative [2] which is converted to 3-(N-acylamine)-quinazoline-4(3H)-one derivative [3] by adding chloroacetyl chloride then potassium thiocyanate is added to give the tridendate 3-(2-imino-thiozolidin-4-one)-quinozoline-4-(3H)-one derivative [4]
-
One mole of benzoxazine derivative was added to one mole of hydrazine hydrate in methanol under reflux for 1 h then filtration and recrystallization using ethanol was performed to give product [2].
-
One mole of product [2] was stirred with one mole of chloroacetyl chloride in DMF for 3 h after that the mixture was poured on crushed ice and product [3] was obtained by filtration and recrystallization in ethanol.
-
One mole of product [3] was refluxed in DMF with potassium thiocyanate for 3 h and poured on crushed ice in presence of hydrochloric acid. The tridendate ligand [4] was obtained by filtration and recrystallization in acetic acid with melting point 140 °C.
Chemical analyses
-
IR spectrum of the tridentate ligand showed absorption bands at 3448 cm−1 (NH), 3093 cm−1 (CH-arom.), 1743, 1671 cm−1 (C=O), 1609 cm−1 (C=N),
-
1HNMR spectrum of the tridentate ligand in (DMSO-d6) revealed signals at
-
δ = 2.36 (s, 3H, CH3), 4.05 (s, 2H, CH2, thiazole), 7.41 (d, 1H, CH-C), 8.07 (d, 1H, CH-b),
-
8.36 (s, 1H, CH-a), 12.61 (br, 1H, NH),
-
The mass spectrum of the tridentate ligand showed a molecular ion peak at m/z = 400 (40.7%) and base peak at m/z = 353 (100%). Other significant peaks were observed at m/z: 327 (12.6%), 326 (6.5%), 116 (12.6%) and 75 (43.0%).
Synthesis of N-(4-chlorophenyl)-2-imino-2H-chromene-3-carbothioamide (B)
The process of synthesis depends on the reaction of N-(4-chlorophenyl) cyanothioacetamide with salicylaldehyde in presence of ammonium acetate giving the required N-(4-chlorophenyl)-2-imino-2H-chromene-3-carbothioamide.
-
One mole of N-(4-chlorophenyl) cyanothioacetamide was refluxed for 3 h with one mole of salicylaldehyde in presence of ammonium acetate then recrystallization using ethanol was performed to give the final product with melting point 180 °C.
Chemical analyses
-
IR spectrum of thioamide tridentate ligand showed absorption bands at 3306 cm−1 (NH), 3000 cm−1 (CH-aromatic), 1586 cm−1 (C=N).
-
1HNMR spectrum of thioamide tridentate ligand in (DMSO-d6) revealed signals at δ = 7.22–8.01 (m, 8H, aromatic H), 8.56 (s, 1H, cromene), 9.8–9.6 (2 s, 2H, 2NH).
-
The mass spectrum of thioamide tridentate ligand showed a molecular ion peak at m/z = 314 (52.5%) and base peak at m/z = 313 (M − 1). Also significant peaks were observed in the spectrum at m/z = 281 (49.1%), 246 (15%), 171 (47.0%) and 118 (43.0%).
Synthesis of 99mTc complexes at tracer level
First, 125 mg of glucoheptonate was dissolved in 50 mL water, then 1 N HCl solution of 50 mg SnCl2 · 2H2O was added to the solution and the pH was adjusted to 7.5. After filtration through a 0.22 μm millipore filter, the solution was divided into 50 vials and lyophilized.
The complex was prepared by ligand exchange reaction using 99mTc(V)O-glucoheptonate as precursor and equimolar quantities of the two ligands. A glucoheptonate kit was reconstituted with 99mTc-pertechnetate solution (20–50 mCi). To an equal molar (1 × 10−6–1 × 10−8 mol) mixture of monodentate ligand and tridentate ligand in ethanol solution, 20–50 mCi (740–1850 MBq) of the 99mTc-glucoheptonate precursor (radiochemical purity >95%) was added. The mixture was agitated in a vortex mixer and left to react in a water bath at 70 °C for 30 min [26]. The reaction time, pH, and temperature of the reaction system were optimized to achieve a high labeling yield.
The 99mTc complex was extracted three times with 2 mL of organic solvent (chloroform) and the organic phase was separated. The percent radiochemical yield of the 99mTc-complex was estimated by determination of the activity in the organic phase related to free 99mTc-pertechnetate in the aqueous phase.
Radiochemical purity
The radiochemical purity of the organic extract was determined by thin layer chromatography (TLC) and high performance liquid chromatography (HPLC):
TLC analysis
The radiochemical purity of the 99mTc complexes were determined by thin layer chromatography-silica gel (TLC-SG). (TLC-SG) sheets were marked 2 cm from the base and lined into fragments 1 cm each up to 14 cm using non-pointed pencil. A spot (5 µL) from the organic phase was applied using micropipette, and then the sheet was developed in an ascending manner in a closed jar contains the developing solvent of CHCl3:CH3OH = 9:1 (v/v) [13]. The sheets after complete development, were removed, dried, and cut into strips, each strip is 1 cm width, then the strip was counted in a well type γ-counter, free 99mTcO− 4 moves with the solvent front (R f = 0.8–1) while 99mTc-complexes remain at the starting line (R f = 0–0.1).
HPLC analysis
To perform the HPLC analysis of the labeled compound, monodentate and tridentate cold solutions were injected into the column (RP18—250 × 4 mm, 5 μm, Lischrosorb) build in HPLC Shimadzu model consisting of pumps LC-9A with a Rheohydron injector and UV spectrophotometer detector (SPD-6A) adjusted to the wave length 254 nm. The column was eluted with the isocratic solvent methanol:water ratio (70:30) and the flow rate was adjusted to 1 mL/min. Then 10 μL of the organic phase, containing 99mTc-complexes, were injected into the column of HPLC and the fractions of 1 mL were collected and counted using NaI(TI) well crystal coupled to SR-7 scaler ratemeter.
Determination of the partition coefficient for 99mTc complexes
The partition coefficient was determined by mixing the 99m Tc-complexes with equal volumes of 1-octanol and phosphate buffer (0.025 M at pH 7.4) in a centrifuge tube. The mixture was vortexed at room temperature for 1 min and then centrifuged at 5,000 rev./min for 5 min. Subsequently 100 μL samples from the 1-octanol and aqueous layers were pipetted into other test tubes and counted in a gamma counter. The measurement was repeated three times. The partition coefficient value was expressed as log p [13], which was found to be equal to 1.7 ± 0.2 and 1.5 ± 0.1 for 99mTc complexes A and B, respectively, showing that the two complexes are good lipophilics and possibly cross the intact blood-brain barrier (BBB).
Biodistribution study of 99mTc complexes
Twelve normal female Albino mice (weighing 20–25 g) were divided into four groups. Each mouse was injected with 99mTc-complex (3–5 MBq/100 μL saline solution containing the 99mTc complex) in the lateral tail vein. Mice were sacrificed at 5, 30, 60, and 120 min post-injection. Blood samples were collected at the time of decapitation. Organs were dissected, weighed, and their radioactivity was measured using a well-type NaI scintillation detector. Samples of fresh blood, bone and muscle were collected in pre-weighed vials and counted. Blood, bone and muscles were assumed to be 7%, 10% and 40%, respectively [27], of the total body weight. Whole brains were removed and frozen for an hour. The organs were weighed and the radioactivity was assessed radiometrically. The results were expressed as % dose/organ and % dose/g. The brain/blood ratio was calculated from the corresponding percentage of the injected dose per gram (% ID/g) values.
Results and discussion
Effect of reaction time
The effect of the reaction time on the radiochemical yield of 99mTc-complexes were studied as indicated in Fig. 4. The reaction mixture was incubated in a water bath at 70 °C for different time periods, ranging from 5 to 60 min. It is clear from Fig. 4 that at 5 min reaction time the radiochemical yield was low (45.3% for 99mTc complex A and 32.5% for 99mTc complex B) because the time is not sufficient for the transfer of reduced technetium from 99mTc(V)O-glucoheptonate complex to the monodentate and tridentate ligands, while by increasing the reaction time up to 30 min the radiochemical yield increased to 80% for 99mTc complex A and 75% for 99mTc complex B which are the maximum yields for the two complexes. With further increase in reaction time up to 45 min, no increase in the radiochemical yields were observed.
Effect of pH of the reaction system
The pH of the reaction medium is a very critical factor; the optimum pH was found to be 7 which gives a yield of 80% for complex A and 75% for complex B. The data presented in Fig. 5 reflects the results obtained from the preparation of the 99mTc-complexes at different pH values using buffer systems with a range from pH 2 to pH 11. The results confirmed the influence of pH of the reaction mixture on the radiochemical yield of the 99mTc-complexes. The percentage of 99mTc-complexes increased gradually with the increase in pH up to 7 reaching a maximum yield. On increasing the pH of the reaction medium above pH 7, the yield of 99mTc-complex A decreased to 59.1% and 48.2% at pH 9 and 11, respectively. The same behavior was observed for 99mTc-complex B at alkaline medium. This may be attributed to the fact that the reduction at high pH usually does not release all oxygen atoms in the pertechnetate molecule, leading to complexes with a TcO3+ core [28].
Effect of temperature of the reaction system
The reaction temperature plays an important role in the labeling process. The reaction mixture was heated at different temperatures ranging from 25 °C to 100 °C for 30 min. The results were presented in Fig. 6. As observed from these data, there is a significant effect of the reaction temperature on the percent labeling yield. When the reaction was performed at room temperature (25 °C) the radiochemical yield was low, but by increasing the reaction temperature to 50 °C the radiochemical yield increased. Maximum radiochemical yields of 99mTc-complexes were obtained at 70 °C reaction temperature. Also, it is clear from this result that the radiochemical yield decreased by increasing the reaction temperature reaching (57.1% for complex A and 52.1% for complex B) at 100 °C. This decrease in the radiochemical yield at high temperatures may be due to the decomposition of the 99mTc-complexes.
HPLC analysis of the 99mTc-complexes
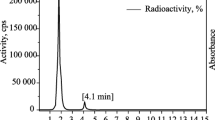
The retention times of the non labeling monodentate ligand, tridentate ligand A and tridentate ligand B were 4, 6 and 7 min, respectively. The radiochromatogram for each complex shows two peaks one at fraction No. 2 which corresponds to the free pertechnetate, while the second peak appears at fraction No. 9 which corresponds to 99 mTc-complex A and fraction No. 8 which corresponds to 99 mTc-complex B which is in coincidence with the UV signal. The tracer was separated by HPLC as shown in Figs. 7 and 8. The fractions were collected and sterilized by Millipore filter (0.22 µm) under aseptic conditions.
Stability
The in vitro stability of 99mTc-complexes were studied and the experiment was carried out for different time periods ranging from 1 to 12 h at room temperature. From the results of this experiment, it can be deduced that the both complexes were stable (radiochemical purity >90%) after being purified by HPLC. 99mTc-complexes display excellent in vitro stability with no decomposition observed during these time periods at room temperature.
Biodistribution
Two in vivo evaluations of the 99mTc complexes were performed during this study, the first one was the investigation of blood-brain barrier penetration, and the second was the biodistribution in different organs in normal healthy mice. The studies were carried out on normal mice weighing (20–25 g). The in vivo behavior of the 99mTc complexes were evaluated in mice at 5, 30, 60, and 120 min post-intravenous injection. Tables 1 and 2 show the results expressed as % ID/total organ in the most relevant organs for complex A and complex B, respectively. The 99mTc complexes, after an intravenous injection, were able to cross the blood brain barrier, resulting in a significant initial brain uptake (1.8% for complex A and 1.4% for complex B at 5 min post-injection) and a good retention (0.6% for complex A and 0.7% for complex B at 120 min) observed in mice. In addition, the ratio of the brain to the blood, expressed as percentage uptake/g tissues, has increased gradually as time passes, which may be due to the rapid clearance of the tracer from the blood. The ratio (brain/blood) was 0.52 after 120 min post-injection for complex B. The washout of activity from the brain was faster during the first 30 min for the complex A, compared to the washout from 30 to 60 min. The complexes have high initial blood, muscle and liver uptake as expected for lipophilic compounds. Nevertheless, the blood and the muscle clearance is quite fast. Excretion occurs mainly through the hepatobiliary tract compared with urinary elimination.
Conclusion
In this work our attempt to synthesize 99mTc-complexes as potential agents for imaging brain receptors has been focused on three basic tasks: synthesis, characterization and evaluation.
The 3 + 1 concept for the preparation of neutral, lipophilic, and small size oxotechnetium complex has been applied in the development of novel diagnostic or therapeutic radiopharmaceuticals. In an effort to develop 99mTc-based radioligands for brain receptors, the “3 + 1” concept was applied and oxotechnetium complexes of the general formula TcO[NN(R)N][N] and TcO[NS(R)N][N] were successfully synthesized and characterized. In general, the preparation of the 3 + 1 complex requires the simultaneous action of a tridentate ligand, containing the NNN or NSN donor atom sets and a monodentate co-ligand, on a suitable oxotechnetium (V). The complex was prepared by ligand exchange reaction using 99mTc(V)O-glucoheptonate as precursor and equimolar quantities of the two ligands. The mixture was left to react in a water bath at 70 °C for 30 min at pH7. This reaction system achieved high labeling yields (75% and 80%). The 99mTc-complexes were able to cross the blood-brain barrier and displayed good initial brain uptake in healthy mice (4.5% ID/g and 3.5% ID/g for complex A and complex B at 5 min post-injection) indicating their suitability for brain receptor imaging.
References
Nunn, A.D.: Radiopharmaceuticals. Chemistry and Pharmacology. Marcel Dekker, Inc., New York (1992)
Hom, R.K., Katzenellenbogen, J.A.: Technetium-99m-labeled receptor specific small-molecule radiopharmaceuticals: recent developments and encouraging results. J. Nucl. Med. Biol. 24, 485–498 (1997)
Jurisson, S.S., Lydon, J.D.: Potential technetium small molecule radiopharmaceuticals. Chem. Rev. 99, 2205–2218 (1999)
Liu, S., Edwards, D.S.: 99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chem. Rev. 99, 2235–2268 (1999)
Pietzch, H.J., Spies, H., Hoffmann, S.: Lipophilic technetium complexes (VI) neutral oxotechnetium(V) complexes with monothiole/tridentate dithiole coordination. Inorg. Chim. Acta 165, 163–176 (1989)
Spies, H., Fietz, T., Pietzch, H.J., Johannsen, B., Leibnitz, P., Reck, G., Scheller, D., Klostermann, K.: Neutral oxorhenium(V) complexes with tridentate dithiolates and monodentate alkane- or arenethiolate coligands. J. Chem. Soc. Dalton Trans. 2277, 77–80 (1995)
Mastrostamis, S.G., Papadopoulos, M.S., Pirmettis, I.C., Paschali, E., Varvarigou, A.D., Stassinopoulou, C.I., Raptopoulou, C.P., Terzis, A., Chiotellis, E.: Tridentate ligands containing the SNS donor atom set as a novel backbone for the development of technetium brain-imaging agents. J. Med. Chem. 37, 3212–3228 (1994)
Pirmettis, I.C., Papadopoulos, M.S., Chiotellis, E.: Novel 99mTc aminobisthiolato/ monothiolato “3 + 1” mixed ligand complexes: structureactivity relationships and preliminary in vivo validation as brain blood flow imaging agents. J. Med. Chem. 40, 2539–2549 (1997)
Spies H., Pietzch, H.J., Johannsen, B.: Technetium, Rhenium and Other Metals in Chemistry and Nuclear Medicine, vol. 5, pp. 101–108. SGE, Padova, Italy (1999)
Johannsen, B., Scheunemann, M., Spies, H., Brust, P., Wober, J., Syhre, R., Pietzch, H.J.: Technetium(V) and Rhenium(V) complexes for 5-HT2A serotonin receptor binding: structure affinity considerations. J. Nucl. Med. Biol. 23, 429–438 (1996)
Meegalla, S., Plossl, K., Kung, M.P., Stevenson, D.A.: First example of a Tc complex as a dopamine transporter imaging agent. J. Am. Chem. Soc. 117, 11037–11048 (1995)
Pirmettis, I.C., Patsis, G., Pelecanou, M., Tsoukalas, C., Papadopoulos, A., Raptopoulou, C.P., Terzis, A., Papadopoulos, M., Chiotellis, E.: Synthesis of oxorhenium(V) and oxotechnetium(V) [SN(R)S/S] mixed ligand complexes containing a phenothiazine moiety on the tridentate SN(R)S ligand. J. Bioorg. Med. Chem. Lett. 11, 1859–1862 (2001)
Liu, F.E.I., Youfeng, H.E., Luo, Z.: Development of 99mTc agents for imaging central neural system receptors, Technical Reports Series No. 426, pp. 37–52. IAEA, Austria (2004)
Kung, H., Bradshaw, J., Chumpradit, S., Zhuang, Z.P., Kung, M.P., Mu, M., Frederick, D.: New TcO(III) and ReO(III) N2S2 complexes as potential CNS 5-HT1A receptor imaging agents. In: Nicolini, M., Bandoli, G., Mazzi, U. (eds.) Technetium and Rhenium in Chemistry and Nuclear Medicine, pp. 293–298. SGE, Padova, Italy (1995)
Mahmood, A., Kronauge, J., Barbarics, E., Freiberg, E., Madras, B., Li, J., Davison, A., Jones, A.: In: Nicolini, M., Mazzi, U. (eds.) Technetium, Rhenium and Other Metals in Chemistry and Nuclear Medicine, pp. 393–399. SGE, Padova, Italy (1999)
Possl, K., Zhuang, Z.P., Meegalla, S.K., Bradshaw, J., Frederick, D., Stevenson, D.A., Kung, M.P., Kung, H.F.: Tc-99m[MPP] complexes: potential 5-HT1A receptor imaging agents. J. Label. Compd. Radiopharm. 37, 306–308 (1995)
Vanbilloen, H., Cleynhens, B., Crombez, D., Verbruggen, A.: In: Nicolini, M., Mazzi, U. (eds.) Technetium, Rhenium and other Metals in Chemistry and Nuclear Medicine, pp. 479–484. SGE, Padova, Italy (1999)
Drews, A., Heimbold, I., Hall, H., Halldin, C., Pietzsch, H.J., Syhre, R., Seifert, S., Brust, P., Johannsen, B.: Autoradiographical evaluation of novel high-affinity Tc-99m ligands for the serotonin-HT1A receptor. J. Label. Compd. Radiopharm. 44, 544–546 (2001)
León, A., Rey, A., Mallo, L., Pirmettis, I., Papadopoulos, M., León, E., Pagano, M., Manta, E., Incerti, M., Raptopoulou, C., Terzis, A., Chiotellis, E.: Novel mixed ligand technetium complexes as 5-HT1A receptor imaging agents. J. Nucl. Med. Biol. 29, 217–226 (2002)
Caraceni, A., Zecca, E., Bonezzi, C.: Gabapentin for neuropathic cancer pain: a randomized controlled trial from the Gabapentin Cancer Pain Study Group. J. Clin. Oncol. 22, 2909–2917 (2004)
Maneufa, Y.P., Luob, Z.D., Leed, K.: Mechanism of action of gabapentin in the treatment of pain. Semin. Cell Dev. Biol. 17, 565–570 (2006)
Gackenheimer, S.L., Schaus, J.M., Gehlert, D.R.: [3H]-quinelorane binds to D2 and D3 dopamine receptor in the rat brain. J. Pharmacol. Exp. Ther. 274(3), 1558–1565 (1995)
Wang, F., Langley, R., Gulten, G., Lynn, G., Gurdyal, S., James, C.: Mechanism of thioamide drug action against tuberculosis and leprosy. J. Exp. Med. 204, 73–78 (2007)
Amer, K.S.: Advances in assessment, diagnosis, and treatment of hyperthyroidism in children. J. Pediatr. Nurs. 20, 119–126 (2005)
Papadopoulos, M., Pirmettis, I., Tsoukalas, C., Nock, B., Maina, T., Raptopoulou, C.P., Terzis, A., Friebe, M., Spies, H., Johannsen, B., Chiotellis, E.: Study on the formation of mixed ligand oxorhenium and oxotechnetium complexes (SNS/S combination). Inorg. Chim. Acta 25, 1–8 (1999)
Pirmettis, D., Papagiannopoulou, M., Papadopoulos E.: Development of 99mTc agents for imaging central neural system receptors, Technical Reports Series no. 426, pp. 77–101. IAEA, Austria (2004)
Rhodes, B.A.: Considerations in the radiolabeling of albumin. Sem. Nucl. Med. 4, 281 (1974)
Abrams, M.J., Larsen, S., Zubieta, J.: Investigations of the technetium hydazo core synthesis and structure characterization of [(N-C4H9)4N][Tc2(NNPH2)2(C6Cl4O2)4]. CH2Cl2·2CH3OH, a Tc(V)/Tc(IV) catecholate complex with the hydrazido ligands adopting the unusual eta-1 bridging mode. Inorg. Chem. 30, 2031–2035 (1991)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amin, A.M., Abou Zid, K., Bayoumi, N.A. et al. Organic synthesis and biological evaluation of novel “3 + 1” mixed ligands of technetium-99m Gabapentin as receptor imaging agents. J Radioanal Nucl Chem 283, 55–62 (2010). https://doi.org/10.1007/s10967-009-0059-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0059-x