Abstract
A new formulation of a freeze-dried kit for the labeling of tetrofosmin with technetium-99m has been developed. The kit contains lyophilized mixture of 0.320 mg tetrofosmin [6,9-bis(2-ethoxyethyl)-3,12-dioxa-6,9-diphosphatetradecane], 0.025 mg stannous chloride dihydrate, 5 mg sodium tartrate and 5 mg sodium hydrogen carbonate. The product contains no antimicrobial preservative. When 99mTc pertechnetate up to 6 mL saline containing 200 mCi is added to lyophilized mixture, a lipophilic, cationic 99mTc complex is formed, 99mTc-tetrofosmin. The performance of newly developed kit is compared with commercially available MYOVIEW kit for heart imaging. The patient studies show that the images of heart obtained by 99mTc-tetrofosmin prepared by new formulation are equally good to MYOVIEW.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial perfusion scintigraphy has now become an established method of diagnosing and evaluating coronary artery disease (CAD). Despite the established clinical value of thallium-201 (201Tl), since its first use in 1975, the physical characteristics of the agent have been considered suboptimal for scintillation camera imaging. Cost of production of 201Tl using a cyclotron and its supply to remote hospitals are also serious issues. To circumvent this problem, several Technetium-99m (99mTc) labeled agents have been developed. The potential advantages of these agents include, optimal photon energy (140 keV) of 99mTc, shorter physical half-life of 6 h allows injection of a higher (10 times) dose, which improves image quality. The freeze dried kits can be reconstituted with desired amount of 99mTc, eluted from 99Mo/99mTc generators in a hospital.
Of the 99mTc-labeled agents, sestamibi and tetrofosmin have gained clinical popularity [1–3]. The clinical applications have expanded from diagnosis to risk stratification of CAD and assessment of a variety of clinical conditions, such as myocardial infarction, reperfusion, and revascularization. Lyophilized kits of sestamibi and tetrofosmin as CARDIOLITE® and MYOVIEW™ are commercially available.
The leaflet of MYOVIEW™ describes the following characteristics [4]. Myoview is supplied as a package of five multidose vials for use in the preparation of a 99mTc tetrofosmin intravenous injection to be used for scintigraphic imaging studies for the delineation of regions of reversible myocardial ischemia in the presence or absence of infracted myocardium. Each vial contains a pre-dispensed, sterile, non-pyrogenic, lyophilized mixture of 0.23 mg tetrofosmin [6,9-bis(2-ethoxyethyl)-3,12-dioxa-6,9-diphosphatetradecane], 30 μg stannous chloride dihydrate (minimum stannous tin 5.0 μg; maximum total stannous and stannic tin 15.8 μg), 0.32 mg disodium sulphosalicylate, 1.0 mg sodium d-gluconate and 1.8 mg sodium hydrogen carbonate. The lyophilized powder is sealed under a nitrogen atmosphere with a rubber closure. The product contains no antimicrobial preservative. When sterile, pyrogen-free sodium pertechnetate 99mTc in isotonic saline is added to the vial, a 99mTc complex of tetrofosmin is formed. The pH of the reconstituted vial is 7.5–9.0. For stress (either exercise- or dipyridamole-induced) and rest imaging, Myoview is administered in two doses. The first dose of 185–300 MBq (5–8 mCi) is given at peak stress, while the second dose of 550–900 MBq (15–24 mCi) is given approximately 4 h later, at rest.
The volume of (diluted) technetium Tc-99m generator eluate added to the vial must be in the range of 4–8 mL. The radioactive concentration of the (diluted) 99mTc generator eluate must not exceed 1.1 GBq/mL (30 mCi/mL) when it is added to the vial. The radiochemical purity of at least 90% prior to administration to patients in clinical studies is mandatory.
In this work formulation of a freeze-dried kit for the preparation of 99mTc-tetrofosmin is reported. The performance of newly formulated kit is compared with commercially available kit of MYOVIEW used for heart imaging.
Experimental
Materials and methods
Tetrofosmin (1,2-bis bis(2-ethoxyethylphosphine) ethane), was obtained from ABX advanced biochemical compounds, Radeberg, Germany. Rats (Sprague–Dawley) were obtained from National Institute of Health (NIH) Islamabad. The Animal Ethics Committee of the Institute gave an ethical approval for the animal experiments. Technetium-99m was obtained from locally produced fission based PAKGEN 99Mo/99mTc generator. All the chemicals used were AR grade and purchased from E. Merck,
Labeling of tetrofosmin with 99mTc
Various parameters such as ligand concentration, stannous chloride concentration, reaction rate, stability of complex, concentration of transchelating agent, eluent volume were optimized to achieve maximum complexation of 99mTc with tetrofosmin. All the experiments were carried out at room temperature 23 ± 2 °C.
Radiochemical analysis
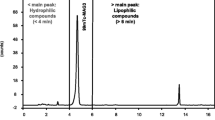
An assay of the radiochemical purity of the prepared 99mTc-tetrofosmin was performed using the following chromatographic procedure. A mixture of 35:65 acetone:dichloromethane was poured into the chromatography tank to a depth of 1 cm. The tank was covered with a lid and allowed to equilibrate with the solvent vapor. ITLC/SG strip was marked with a pencil line at 3 cm from the bottom and, using an ink marker pen, at 15 cm from the pencil line. The pencil line indicates the origin where the sample is to be applied and movement of color from the ink line will indicate the position of the solvent front when upward elution should be stopped. Cutting positions at 3 cm and 12 cm above the origin (Rf 0.2 and 0.8, respectively) was also marked in pencil. Using a 1 mL syringe and 22–25 G needle, 10–20 μL sample of the prepared 99mTc-tetrofosmin was applied at the origin of the ITLC/SG. Then it was placed in the chromatography tank immediately and cover was replaced. It was ensured that the strip did not adhere to the walls of the tank. When the solvent reached the ink line, the strip was removed from the tank and allowed it to dry. The strip was cut into three pieces at the marked cutting positions and activity was measured in a gamma counter. The radiochemical purity of 99mTc-tetrofosmin was calculated. Free 99mTc-pertechnetate runs to the top piece of the strip, while 99mTc-tetrofosmin runs to the center piece of the strip. Reduced hydrolyzed 99mTc and any hydrophilic complex impurities remain at the origin in the bottom piece of the strip.
An alternate method, Whatman 31 ET Chr paper as a stationary phase and ethyl acetate as a mobile phase was also used for the determination of radiochemical purity of 99mTc-tetrofosmin. This system involves the use of a regular Whatman 31 ET Chr strip, 1 cm × 9.0 cm, with the origin, cut line, and solvent front at 1.0 cm, 2.5 cm, and 7.0 cm from the bottom of the strip, respectively. A 10–20 μL drop of 99mTc-tetrofosmin was placed at the origin, 1 cm from the bottom of the strip. The strip was then placed in an ethyl acetate developing chamber. The bound 99mTc-tetrofosmin migrated to the top of the strip, while any 99mTc impurities remained at the bottom. The distribution of radioactivity on chromatographic strips was also measured sometimes by 2π Scanner (Berthold, Germany).
Charge determination by electrophoresis
Electrophoresis was performed on Whatman 1 paper strip impregnated with the electrolyte solution and at a potential difference of 300 V. Electrolyte used was mixture of 0.07 M (pH 6.4) phosphate buffer: 0.9% NaCl: 95% ethanol (1:2:2). The analysis was run for 120 min. The developed electrophoresis strips were left dry, and each of them was divided into 1 cm parts. The quantification of radioactivity was carried out by gamma counter.
Lipophilicity
The lipophilicty of the 99mTc-tetrofosmin complexes with a radiochemical purity of at least 90% was determined by partitioning the complexes between chloroform and water and counting the activity in both phases in a test tube. The test tube is vortexed at room temperature for 1 min and then centrifuged for 5 min. A 0.5 mL aliquot of both phases is pipetted into separate test tubes and counted in a well counter. The partition coefficient was calculated.
Biodistribution studies
Biodistribution studies were performed in Sprauge–Dawley rats (200–250 g) procured from the animal house of National Institute of Health Islamabad, Pakistan. An amount of 0.1–0.2 mL of the preparation (370–550 kBq) was injected into the tail vein of rats, anesthetized with diethyl ether. At 2 min, 60 min and 24 h postinjection the animals were killed by cervical dislocation. Blood and urine samples were collected. For calculation of blood activity, the blood volume was assumed to be 6% of the animal body weight. Other organs were removed, rinsed with saline, and blotted dry to remove the residual blood. Results were expressed as percent dose/organ by counting the samples in a gamma counter against the suitably diluted aliquots of the injected solution as standard.
Clinical evaluation
A total of 10 patients referred for cardiac study were evaluated using one day protocol (Rest followed by Stress); 8–10 mCi of 99mTc-tetrofosmin was injected to the patients. Imaging was started with in 5 min of injection including in the view lungs, heart, liver, kidneys and part of intestine. A frame rate of 2 min per view 128 × 128 matrix for 30 min, and delayed images at 60 min, 90 min and 120 min were acquired.
Results and discussion
In a search for 99mTc radiopharmaceuticals that provide diagnostic information compatible to those of 201Tl, efforts were made to synthesize cationic 99mTc complexes with appropriate lipophilicity so that the complexes can reflect myocardial blood flow. Jones et al. prepared organometallic 99mTc(I) hexakis(isonitrile) complexes [1]. After extensive structure-distribution studies of the isonitrile derivatives, hexakis(2-methoxy-isobutylisonitrile; MIBI) technetium(I) was found to improve the biodistribution properties when compared with the prototype compound, hexakis(t-butylisonitrile) technetium(I), due to rapid clearance of radioactivity from the liver and lung [2]. Kelly et al. [3]. synthesized cationic and lipophilic 99mTc complex of diphosphine ligand, 1,2-bis[bis(2-ethoxyethylene)phosphino]ethane (tetrofosmin) with a 99mTcO2 core as shown in Fig. 1. This complex is now approved as a radiopharmaceutical for myocardial blood flow. Freeze dried kits of MIBI and Tetrofosmin are sold as Cardiolite and Myoview, respectively, in the market for heart and breast scintigraphy. The reconstitution of tetrofosmin is simpler than MIBI, since no heating is required to make 99mTc-tetrofosmin.
The complexation parameters were varied with a view to optimize the labeling yields (an average value obtained from at least five sets of experiments). The reaction mixture volume was kept 1.2 ± 0.2 mL. The two methods of ascending chromatography used for the quantification of 99mTc-tetrofosmin gave similar results (±1.5%).
In preliminary experiments the quantity of ligand (tetrofosmin) was kept 230 μg as in MYOVIEW formulation; however, the amount was not sufficient to give >90% labeling efficiency in newly developed formulation, hence it was increased to 320 μg, which gave consistently higher labeling yield, whenever various parameters were changed. Since transchelating agent disodium sulphosalicylate was not available from commercial sources in Pakistan, it was replaced by tartrate in our experiments.
Effect of concentration of stannous chloride
Radiochemical yield increased with the increase of stannous chloride dihydrate amount in reaction mixture (Fig. 2). The labeling yield was improved from 70% to more than 90% by increasing the concentration of stannous chloride dihydrate from 10–20 μg with 320 μg of tetrofosmin. Up to 30 μg stannous chloride dihydrate labeling efficiency remained more than 90%, however, it decreased with the addition of more reducing agent. Therefore, concentration of stannous chloride was fixed at 25 μg and maximum labeling yield was obtained.
Effect of transchelating agent
Figure 3 shows the effect of transchelating agent on the labeling efficiency of 99mTc-tetrofosmin. Addition of 1–4 mg Sodium tartrate increased the labeling yield of 99mTc-tetrofosmin from 50 to 90%. More than 90% 99mTc-tetrofosmin was found after the addition of 5 or 6 mg sodium tartarate, hence 6 mg of transchelating agent was used in the final formulation. In MYOVIEW kit two transchelator, gluconate and disodium sulphosalicylate are used.
Rate of complexation and stability of 99mTc-tetrofosmin
Although no heating is required for the labeling of tetrofosmin with 99mTc, but 15 min incubation period at room temperature is mandatory to obtain more than 90% radiochemical purity of 99mTc-tetrofosmin. Sometimes after few minutes of reconstitution more than 90% labeling yield was obtained, however, only incubation period of 15 min gave a reliable labeling efficiency of 99mTc-tetrofosmin. The complex of 99mTc-tetrofosmin was found stable up to 5 h at room temperature as shown in Fig. 4.
Effect of 99mTc eluate volume on freeze dried kit labeling
The concentration of 99mTc decreases after every day with the arrival of 99Mo/99mTc generator in the hospital, therefore effect of eluate volume on labeling efficiency of 99mTc-tetrofosmin in newly formulated kit was studied. More than 90% labeling efficiency of 99mTc-tetrfosmin was obtained from 1–6 mL addition of eluate in the freeze dried kit (Fig. 5). A shelf life of 2 months (study period) was found for the newly formulated kit of tetrofosmin.
Lipophilicity and charge of complex
Figure 6 shows the radiochromatogram of electrophoresis, which confirms that the complex of 99mTc-tetrofosmin is positively charged. Quantitative extraction of 99mTc-tetrofosmin was observed in chloroform, showing lipophilic properties of the complex.
Formulation and reconstitution of tetrofosmin freeze dried kit
In newly formulated kit, each vial contains a predispensed, sterile, non-pyrogenic, lyophilized mixture of 0.320 mg tetrofosmin [6,9-bis(2-ethoxyethyl)-3,12-dioxa-6,9-diphosphatetradecane], 0.025 mg stannous chloride dihydrate, 5 mg sodium tartrate and 5 mg sodium hydrogen carbonate. The lyophilized powder is sealed under a nitrogen atmosphere with a rubber closure. The product contains no antimicrobial preservative. When 99mTc pertechnetate up to 6 mL containing 200 mCi is added to lyophilized mixture, a lipophilic, cationic 99mTc complex 99mTc-tetrofosmin is formed. The pH of the final product is ~9.
Animal biodistribution
Table 1 shows the comparison of biodistribution of MYOVIEW and 99mTc-tetrofosmin prepared from locally developed kits in male Sprague Dawley rats. The complex obtained by newly formulated kit shows good heart uptake with rapid clearance from blood and liver, and there is no significant uptake in lung tissues. The data are in good agreement with results obtained with MYOVIEW formulations.
Clinical evaluation
Studies in normal volunteers have demonstrated rapid myocardial uptake of 99mTc-tetrofosmin, and rapid blood, liver and lung clearances. Uptake in the myocardium reaches a maximum of about 1.4% of the injected dose (i.d.) at 5 min and approximately 1.2% of the i.d. at 2 h, respectively. Figure 7 shows the heart images (rest) of a patient injected with 99mTc-tetrofosmin prepared by using newly formulated kit. The images are as good as obtained by the commercially available kit of MYOVIEW.
Conclusion
The newly formulated freeze-dried kit for the preparation of 99mTc-tetrofosmin gave similar uptake in animal as well as in patients compared to commercially available MYOVIEW™. The transchelating agent tartrate can be safely used in place of disodium sulphosalicylate and gluconate.
References
Jones, A.G., Davison, A., LaTegola, M.R., Brodack, J.W., Orvig, C., Sohn, M., Toothaker, A.K., Lock, C.J.L., Franklin, K.J., Costello, C.E., Carr, S.A., Biemann, K., Kaplan, M.L.: Chemical and in vivo studies of the anion oxo[N,N'-ethylenebis(2-mercaptoacetimido)]technetate (V). J. Nucl. Med. 23(9), 801–809 (1982)
Piwnica-Worms, D., Kronauge, J.F., Chiu, M.L.: Enhancement by tetraphenylborate of technetium-99m-MIBI uptake kinetics and accumulation in cultured chick myocardial cells. J. Nucl. Med 32(10), 1992–1999 (1991)
Kelly, J.D., Forster, A.M., Higley, B., Archer, C.M., Booker, F.S., Canning, L.R., Chiu, K.W., Edwards, B., Gill, H.K., McPartlin, M., Nagle, K.R., Latham, I.A., Pickett, R.D., Storey, A.E., Webbon, P.M.: Technetium-99m-tetrofosmin as a new radiopharmaceutical for myocardial perfusion imaging. J. Nucl. Med. 34(2), 222–227 (1993)
Product Monograph MYOVIEW™ [Kit for the Preparation of Technetium Tc-99m Tetrofosmin Injection]: GE Healthcare, Medi-Physics, Inc., Princrton, NJ (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pervez, S., Mushtaq, A. Formulation of a freeze-dried kit for the preparation of 99mTc-tetrofosmin. J Radioanal Nucl Chem 281, 371–377 (2009). https://doi.org/10.1007/s10967-009-0010-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0010-1