Abstract
To enhance the EMI shielding capability in the X-band of a polymeric system (poly(vinylidene fluoride-trifluoroethylene)) P(VDF-TrFE) containing f-MWCNTs, magnetic ionic liquid (MIL) (1-butyl-3-methylimidazolium tetrachloroferrate ([Bmim][FeCl4]) has been used in the present study. The porous structure formation due to the incorporation of the magnetic ionic liquid in the polymer has been seen in the scanning electron micrographs while the pore volume and their area has been measured using BET technique. The XRD, FTIR and RAMAN studies have thrown light on the interaction between the different constituents of the polymer composite. The synthesized polymer composite systems with 5 wt% of f-MWCNTs has high conductivity of ~ 0.65 S.cm− 1 and moderate shielding effectiveness of ~ 29.43 dB at 8.2 GHz. The shielding effectiveness has been found to improve to ~ 34 dB on addition of 20 wt% of MIL of which the major contribution is due to the absorption mechanism (absorption loss ~ 24 dB) which is attributed to the dielectric, magnetic and multiple reflection losses between interfaces. The above obtained result suggests that the MIL based polymer composite system having only three constituents, is a promising EMI absorbing material in the X-band due to the contribution of the MIL to both the conductivity and the magnetic loss.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Technologically, the rapid development of communication and its extensive utilization in electronic devices (like local area network, military, radar systems, etc.) has created pollution/noise due to electromagnetic interference (EMI). The over exposure of this pollution is a potential threat for human health as well as for electronic devices [1,2,3]. This is likely to get more serious with the arrival of the 5G era. Hence, an intense effort is required to develop high-performance EMI shielding materials (such as plastics, ceramics, composites and functional materials). A good EMI shield material must have moderate conductivity, high dielectric constant and high magnetic loss. As all these properties cannot be envisaged in a single material, multi-component materials have been pursued. Traditionally, metal-based EMI shielding materials were preferred due to their high electrical conductivity [4, 5]. However, their application was limited due to major drawbacks like corrosion, structural stiffness, high density and most importantly, ample surface reflection which sent back the EM waves in the surroundings/atmosphere. To overcome these problems, shield materials with polymer matrices having mostly carbon based fillers (e.g., multiwall carbon nanotubes, carbon fiber, graphene, and MXene, etc.) [6] were focused upon. Properties of these fillers like high mechanical stability, ultrahigh specific surface area, and high conductivity [7] were the reasons for their preferred usage. Also, magnetic nanoparticles have been used as fillers (e.g., Fe, Co, Ni, ferrite, and alloys) [8] as they provide large magnetic losses. In EMI shielding mechanism, both dielectric and magnetic fillers play significant roles in dissipating EM energy through dielectric and magnetic losses [9, 10]. Kim et al. [11] have reported shielding effectiveness (SE) with MWCNTs containing Fe catalyst in PMMA polymer matrix of ∼27 dB while Che et al. [12] have reported SE of ∼25 dB in Fe encapsulated with CNTs. Kallumottakkal et al. [13] have reported ∼43 dB SE in CoFe in PU matrix.
As MWCNTs possess many exceptional properties like high mechanical strength and conductivity, light weight, low density and high aspect ratio, they are mostly used in the field of EMI shielding [7]. However, the carbon nanofillers have a tendency to aggregate in polymeric matrix due to the strong intrinsic Van der Waals attractive force [14,15,16] making it difficult to disperse. Therefore, for proper dispersion as well as interfacial interaction between MWCNTs and the polymeric matrix, various techniques (e.g., ultrasonication, surface modification) have been used. For surface modification of MWCNTs, chemical functionalization (covalent and non-covalent bonding techniques) and oxidative acid treatments have been reported [17, 18]. This study has used the oxidative acid treatment for functionalization.
It is well known that the absorption of EM waves is based on the change of these waves into heat or another type of energy and/or radiation [19, 20]. This is achieved via the shield materialꞌs electric and/or magnetic dipoles ability to interact with the EM wave. Normally, the polymer matrices are insulating in nature through which the EM waves can pass through. Also, they have low density, can be made very thin and flexible. The ever decreasing size of modern day electronics and telecommunications devices requires flexible materials which can be moulded into desired shapes and sizes. Polymer matrices fulfil all these requirements. The work in this paper is on polymer composites using the polymer polyvinylidene fluoride-trifluoroethylene (P(VDF-TrFE)). P(VDF-TrFE) [21,22,23] has attracted much attention of scientist/technologists as it exhibits piezoelectric, pyroelectric and ferroelectric properties. Many studies are available on PVDF and its copolymers being exploited in modern electronic devices for energy storage and harvesting applications. The five crystalline phases (viz., α, β, γ, δ, and ε) of PVDF can be obtained by varying the synthesis process. Among these, the β-phase has ferroelectric properties. In the case of its copolymer, P(VDF-TrFE), the β-phase being inherently present gives it a ferroelectric nature [24,25,26,27]. The high dielectric constant of (P(VDF-TrFE) 70/30 mol%) makes it a good choice as EMI shielding material.
Another viable novel approach is using magnetic ionic liquids (MILs) in the polymer matrix having MWCNTs. MILs are room temperature ionic liquids (RTILs) and obtained by the incorporation of a paramagnetic component as either the cation or anion in the IL structure [28, 29]. MILs are attracting much attention over the conventional ILs due to their ability to exhibit permanent magnetic response to an external magnetic field without the need of further adding magnetic particles as the magnetic properties originate either from the anion or cation or from both [30,31,32]. The magnetic, electrical, mechanical and electromechanical properties of MILs have been enlisted in an earlier study [33]. These MILs can be used as alternative to magnetic fillers as they have the added advantage of enhancing the mobile charge carrier density (and thereby conductivity) of the material along with the magnetic losses. Kowsari et al. [34] have reported MIL (1-methylimidazolium tetrachloroferrate) conducting GO/epoxy resin composites and found the SE to be ∼ 25.21 dB in X-band.
The main focus of this work is to develop a class of MIL based polymer composites using the host matrix of P(VDF-TrFE) dispersed with f-MWCNTs. EMI shielding materials based on (Poly(vinylidene fluoride-trifluoroethylene) P(VDF-TrFE)/f-MWCNTs/1-butyl-3-methylimidazolium tetrachloroferrate ([Bmim][FeCl4])) have been synthesized and characterized. With the addition of f-MWCNTs in the pristine P(VDF-TrFE), conductivity as high as 0.65 S.cm− 1 has been achieved which is credited to the formation of the connecting pathways by the f-MWCNTs and large interfacial area between the host matrix and the f-MWCNTs. The incorporation of MIL in the above polymer composite imparts it the following properties; (i) provides additional free charge carriers for conduction, (ii) improves magnetic losses, and (iii) enhances the porosity (leading to multiple scattering of EM waves). Here, the addition of 20 wt% of MIL in the polymer composite has given a total shielding effectiveness (SET) of ∼ 34 dB in X-band (corresponding to maximum absorption loss (∼24 dB)). This enhancement in SE can be attributed to the magnetic losses, multiple reflection losses, and dielectric losses.
Experimental section
Preparation of functionalized MWCNTs
Acid treatment has been used for the surface modification of the pristine MWCNTs (50-90 nm diameter and ≥ 95% carbon basis, procured from Sigma Aldrich, USA). Oxygen containing groups (viz., carboxyl groups) have been introduced onto the surfaces of pristine MWCNTs. Initially, the pristine MWCNTs (1.0 g) have been taken in a glass plate and added to a 200ml solution of nitro-sulfuric acid [(3:1 ratio (v:v) mixture of H2SO4 (98%)/HNO3 (68%)]. Thereafter, the above solution has been ultrasonicated (at 40 °C) for 3 h. Then, the mixture has been filtered and washed with deionized water (4-5 times) till a pH of ~ 7 is attained. The obtained product has been dried in a vacuum oven at 70 °C for 24 h. Finally, functionalized MWCNTs (f-MWCNTs) have been obtained. The schematic representation of the above functionalization process has been shown in Fig. 1.
Synthesis of polymer composites
Polymer composites have been prepared using the solution cast technique. In this technique, first, the polymer (P(VDF-TrFE), 70:30 mol in powder form procured from Sigma Aldrich, USA) has been dissolved in acetone at 45 °C and then continuously stirred for 6 h using a magnetic stirrer. The f-MWCNTs dispersed in acetone are then ultrasonicated for 1 h before adding to the polymer solution. This mixture is again stirred for 6 h at 45 °C. The resulting solution is then poured into Petri dishes. The solvent evaporates at room temperature in a few days and a dry film is obtained. The optimized weight% of P(VDF-TrFE):f-MWCNTs has been found to be 1:5 corresponding to the highest EMI shielding property.
In this optimized P(VDF-TrFE):f-MWCNTs mixture, now different amounts (10, 20, and 30 wt%) of the magnetic ionic liquid (1-butyl-3-methylimidazolium tetrachloroferrate) [Bmim][FeCl4] (purity ≥ 98.0%, MW ≈336.88 g mol− 1, purchased from TCI Chemicals (India) Pvt. Ltd) has been added. This mixture has been stirred for 4 h at 45 °C. Like above, the obtained solution has been poured into Petri dishes and left to dry for 3-4 days at room temperature.
Before characterization of the prepared films, they have been vacuum dried at 10− 3 torr for 4-5 days to remove traces of moisture/solvent giving free-standing flexible films. The schematic of the entire procedure is shown in Fig. 2. Also, the codes for the prepared samples along with their respective composition are given in Table 1.
It is to be noted that due to the following two reasons, the MIL remains confined in the polymer matrix: (i) MILs are known to have very high viscosity which helps in containing them within the pores and (ii) when MIL enters the pores, electrostatic interactions occur between the MIL’s ions and the polymeric matrix dispersed with f-MWCNT attributed to the presence of charges in its structure and ease of formation of hydrogen bond (due to the presence of OH− groups and H terminations) and π-π interactions (i.e., cyclic structure and double bonds) [35].
Characterization
Powder XRD data has been obtained using Shimadzu D8 DISCOVER X-ray diffractometer. Raman-scattering measurements have been done (at room temperature) using a Renishaw micro-Raman system with a grating of 2400 lines/mm. Fourier transform infrared (FTIR) spectra have been recorded in transmission mode using PerkinElmer FTIR infrared spectrometer in the range 4000-400 cm− 1 having resolution 2 cm− 1 to investigate the nature of the chemical bonds. Magnetic studies of the MIL and composite films have been investigated (at room temperature) using Vibrating Sample Magnetometer (VSM) (The Microsens ADE-Model EV9) in an external field up to 1 kOe. The surface morphology of the P(VDF-TrFE)/f-MWCNTs/[Bmim][FeCl4] films has been obtained with a Scanning Electron Microscope (SEM, JEOL, JSM 5600) operated at accelerating voltage between 10 and 30 kV. Thermal studies of the polymer composites have been done in N2 atmosphere (at heating rate 10 oC/min) by using Thermo Gravimetric Analysis (TGA) equipment from Perkin Elmer. The ionic conductivity has been determined using Electrochemical Impedance Spectroscopy (EIS) measurements at room temperature. To investigate the electromagnetic shielding parameters, dielectric studies, and magnetic studies in the X-band (8.2–12.4 GHz) at room temperature, Agilent E8362B Vector Network Analyzer has been used. To obtain the EMI shielding parameters and dielectric properties, rectangular strips of polymer composites films have been placed inside the sample holder with dimensions 28 mm x 13 mm x 2.6 mm (length x width x thickness).
Results and discussion
Structural studies and surface morphology of MWCNTs and f-MWCNTs
Figure 3i shows the XRD profiles of pristine MWCNTs and f-MWCNTs. In Fig. 3i, the characteristic peaks for pristine MWCNTs have been observed at 25.99o [002], 43.03o [100] and 54.40o [004] while these peaks appear at 25.44o [002], 43.30o [100] and 54.22o [004], respectively, for f-MWCNTs [36, 37]. The diffraction peaks at 25.9o and at 25.4o both correspond to pristine MWCNTs and f-MWCNTs exhibiting the hexagonal graphite structure [38]. This observation shows that although the acid treatment of pristine MWCNTs does not change its nanotube like structure [38], it does enhance the peak intensity in f-MWCNTs. This increase in intensity shows a highly graphitized (crystalline) structure of the f-MWCNTs as reported earlier [39].
Figure 3iia-b show the surface morphology of acid-treated MWCNTs along with pristine MWCNTs. It can be seen that acid treatment has resulted in the f-MWCNTs having nanotubes which are randomly and loosely entangled while the pristine MWCNTs have a higher level of aggregation. Additionally, the morphology of functionalized MWCNTs is found to remain intact even after acid treatment.
To know the possible conformational changes and to confirm the attachment of functional groups at the surface of MWCNTs, FTIR has been used. The FTIR spectra of (a) MWCNTs and (b) f-MWCNTs have shown in Fig. 3iii. The band at 1745 cm− 1 corresponds to the C = O stretching vibration of –COOH, curve (b), which confirms that the –COOH groups were attached with MWCNTs. The peak at 3423 cm− 1 is ascribed to the –OH stretching of –COOH groups, present on the nanotube surface [40]. The presence of the above functional groups at the surface of f-MWCNTs confirms the surface modification of MWCNTs.
The Raman spectra of the pristine MWCNTs and f-MWCNTs are shown in Fig. 3iv. The characteristic peaks located at ∼1356 cm− 1 (D-band), ∼1582 cm− 1 (G-band), ~ 2454 cm− 1 (D’-band) and ∼2707 cm− 1 (G’-band) of pristine MWCNTs are seen in Fig. 3iva. The D band indicates the amorphous nature of carbon which may be due to the structural defects/ vacancy present in the graphitic structure. The stretching in the graphitic plane which leads to tangential shear mode of carbon atoms is reflected in the G-band [41,42,43] whose strength is either due to the amount of disordered graphite or the conjugation disruption in the graphene sheet [44]. After the functionalization of MWCNTs, the position of the bands (D, G, D’, and G’) is shifted towards higher wave numbers (~ 2-3 cm− 1) as shown in Fig. 3ivb. This shift may be due to the breaking of bonds and/or attached chemical groups which can lead to defects in the structure. These observed shifts in bands once again, confirm that the oxidation groups do not damage the graphitic structure of MWCNTs [45, 46] on functionalization. Also, for quantification of disorder in the structure, the intensity ratio of the D and G bands (ID/IG, i.e., R) has been calculated and given later in Table 2. This value of R is less for MWCNTs in comparison to the f-MWCNTs. The obtained value of R confirms that covalent functionalization results in higher structural defects in the f-MWCNTs.
Structural studies and surface morphology of composite films
XRD studies
Figure 4 shows the XRD patterns of different composite films. In Fig. 4a, the prominent peaks of polymer P(VDF-TrFE) appear at 19.5° [110/220], 34.48o [001], and 40.53o [201] overriding a broad halo in the range 10o-45o which are associated with the β-phase of the polymer [47]. The addition of f-MWCNTs in the P(VDF-TrFE) matrix shows a slight reduction in the intensity of all the peaks along with the appearance of a new peak at 2θ∼27.2o [002], a signature of f-MWCNTs. The minor shift in diffraction peaks may be due to the complex formation between the P(VDF-TrFE) and f-MWCNTs, Fig. 4b-e. Further, with the incorporation of MIL in the PFE5fM film, an additional new peak is seen at 2θ ∼29.45o (JCPDS file No. 00-001-1059) (which is of FeCl3) and marked by ‘*’ in Fig. 4c. However, on further (> 10 wt%) addition of MIL, this peak is found to disappear. This observation can be corroborated with the SEM studies given below. On increasing the MIL content to 30 wt%, there is an increase in the halo region and a reduction in the crystalline peaksꞌ intensity.
Morphological studies
Figure 5 shows that SEM images of pure P(VDF-TrFE), PFE5fM, and polymer composite films with different wt% of MIL. Figure 5a shows the pristine polymer film with voids. The addition of f-MWCNTs to the polymer, Fig. 5b, shows uniform dispersion of the nanotubes (due to ultrasonication before dispersion) as well as enhancement in the voids’ size in the composite films. The f-MWCNTs are seen to be completely wrapped by the polymer. Further, on the introduction of MIL to the polymer composite, Fig. 5c-e, it can see that the voids is maximum for 30 wt% of MIL content and minimum for 20 wt%. It is worth mentioning that the cubes observed in the PFE5fM10MIL polymer composite (Fig. 5c) are that of FeCl3 whose signature is also seen in the XRD pattern (marked by *). The FeCl3 formation is attributed to the reduction of the MILꞌs anions.
The porosity of the polymer composites has been measured by BET technique. Worth noting is the fact that although the pore size is minimum for 20 wt% of MIL, Fig. 5d, the pore area is maximum for this composition (as listed in Table 3). These observations of surface morphology and porosity have resulted in enhanced EMI shielding by films with 20 wt% of MIL which is explained below in detail in the Section of electrical and shielding studies. When MIL is added to the polymer composite system, the molecules of MIL can either get attached at the pore openings or at the surface considerably changing (decreasing/increasing) the pore parameters like surface area, volumes, etc. of the porous material [48].
FTIR analysis
The ATR spectra of pure [Bmim][FeCl4], P(VDF-TrFE), PFE5fM and PFE5fM + x wt% MIL (where, x = 10, 20, and 30) have shown in Fig. 6i-ii. Figure 6i shows the ATR spectra of pure MIL and its characteristic vibrations whose assignments are listed in Table 4 [49, 50]. Figure 6ii shows the ATR spectra of pure P(VDF-TrFE) and its composites. The vibrational bands at 840 cm− 1, 880 cm− 1, 1076 cm− 1, 1165 cm− 1, 1283 cm− 1 and 1402 cm− 1 correspond to the β-phase of P(VDF-TrFE) [51]. With the addition of f-MWCNTs, few β-phase related bands get shifted and become intense. This may be due to the interaction between f-MWCNTs and P(VDF-TrFE) (as already discussed in XRD and SEM).
The incorporation of MIL (in varying amounts) in the polymer composite is shown in Fig. 6iic, 6iid, & 6iie. The MIL related bands get shifted towards lower wavenumbers (by ~ 2-3 cm− 1) with increasing content of MIL in the polymer composite. This shift and their corresponding higher intensity is due to the interaction between the MIL and the P(VDF-TrFE). Also, the band related to the β-phase of the polymer shifts to lower wavenumbers (listed in Table 4) and becomes prominent with increasing MIL content.
Additionally, some of the characteristic peaks of pure MIL at 619 cm− 1, 647 cm− 1, 831 cm− 1, 1105 cm− 1 and 1162 cm− 1 disappear on increasing the MIL content in the polymer composite. This may be due to the complexation between the polymer, f-MWCNTs and MIL [52]. The complexation of f-MWCNTs and the cations/anions of MIL with the polymer backbone is schematically shown in Fig. 7.
Raman studies
Figure 8 shows the Raman peaks of the polymer composites. It is seen that for all the polymer composites containing MIL, the G band shifts towards higher wave number side compared to that of f-MWCNTs while the D band shift towards the lower wave number side (in Table 2). The shifting of both the D and the G band in the Raman spectra is attributed to the wrapped polymer-MIL complex on the nanotube surface. This has been seen in the SEM images also as discussed earlier. Also, the shift in the G band can be due to a change in the amount and type of chemical groups attached to the functionalized MWCNTs surface which changes the electronic structure of the f-MWCNTs [53]. The complexation between the MIL, f-MWCNTs and polymer matrix can affect the density of electronic states of f-MWCNTs hence requiring higher energy for vibrations resulting in Raman peaks at higher frequency [54, 55].
On addition of 5 wt% of f-MWCNTs in the polymer matrix, the value of R increases as disorder (amorphous region) in the composite increases which is evidenced in SEM also (Fig. 5b) [56]. However, a drop in R value has been seen on the addition of 10 wt% of MIL in PFE5fM polymer composite which is indicative of enhancement in crystallinity in these films attributed to the formation of FeCl3 crystals explained above in XRD and SEM sections, Figs. 4c and 5c. On further increasing the MIL content (> 10 wt%) in the composite, the value of R (Table 2) again increases because of increased amorphicity (reinforced by XRD and SEM, Figs. 4d-e and 5d-e. Also, new peaks are seen at 112 cm− 1(Fe-Cl symmetric vibration) and 334.62 cm− 1 (Fe-Cl symmetric stretch) which are the signatures of FeCl4− anions of the MIL [34, 57].
The above obtained results on the polymer composites using XRD, SEM, FTIR, and RAMAN studies are in consonance with each other and further studies given below will throw light on their effective role as EMI shield material.
Conductivity
Figure 9 shows the room temperature (300 K) conductivity of the polymer composites with MIL incorporated in different wt% (i.e., x wt%, where x = 10, 20, and 30). The conductivity varies with MIL content, being maximum for 20 wt% of MIL. It should be noted that the polymer composite films on addition of MIL become mixed, i.e., electron (due to MWCNTs) plus ion (due to MIL) conductors [58]. Liu et al. [59] and De Volder MFL et al. [60] have reported the electrical conductivity of pristine MWCNT to be ∼ 8 × 108 S/cm. The polymer composite film with 5 wt% of f-MWCNTs without MIL shows conductivity of ~ 0.65 mS/cm due to the formation of conducting network by f-MWCNTs in polymer matrix. After the addition of 10 wt% of MIL in the composite (PFE5fM), conductivity slightly decreases (~ 0.52 mS/cm) at ambient temperature due to plasticization effect of MIL (which is also reflected in XRD and SEM) which restricts the polymer chain motion thereby restricting charge carrier movement. On the incorporation of 20 wt% of MIL to the PFE5fM, the conductivity is found to be maximum (∼1.77 mS/cm) as a large number of ions of the MIL become available for conduction. However, on the addition of 30 wt% of MIL to the polymer composite, the conductivity slightly reduces (∼1.24 mS/cm) as the connecting pathway for the charge carriers gets disrupted due to the enhanced pore size of this composite (Fig. 5e) [61].
TGA studies
Figure 10 shows the TGA curves of the P(VDF-TrFE), PFE5fM, and PFE5fM + x wt% MIL (where, x = 10, 20, and 30). The thermal stability of pristine P(VDF-TrFE) is upto ∼410 °C. On the incorporation of f-MWCNTs, the thermal stability further increases to ~ 430 °C. However, with the introduction of MIL in the PFE5fM composite, the thermal stability decreases to ∼350 °C due to the change in the microstructure of the films as MIL addition increases the porosity of the films, destroying the crystalline nature leading to an earlier onset of melting.
All the prepared samples are thermally stable up to ∼350 °C which is sufficient for practical applications.
Worth mentioning is the fact that the weight loss at each step in the multi-component system has not been assigned to individual components as the constituents of the polymer composites have varying interactions with each other thereby making it speculative to interpret precisely.
VSM study
Figure 11 shows the magnetic properties of pure MIL, [Bmim][FeCl4] (inset of Fig. 11) and its composites having different wt% of MIL (10, 20, and 30) (at room temperature). As seen in the inset, the MIL exhibits a linear variation of magnetization with the magnetic field confirming its paramagnetic nature [62]. Similar linear behaviour has been observed for the composite films (with varying MIL content 10–30 wt %). As the MIL content is increased, the saturation magnetization also monotonically increases slightly. Although, the obtained values of saturation magnetization (MS) are low, they are sufficient to contribute to the EMI shielding effectiveness in terms of magnetic losses.
Electrical and shielding studies
Complex permittivity (\({\epsilon }^{*}\)) and permeability (\({\mu }^{*}\)) of polymer composite films are written as
where, \({\epsilon }^{{\prime }}\), \({\mu }^{{\prime }}\) and \({\epsilon }^{{\prime }{\prime }}\), \({\mu }^{{\prime }{\prime }}\), respectively, are storage and loss capability of electric and magnetic energy.
Figure 12i-ii show the frequency dependence of the dielectric constant and dielectric loss of the composite films in the X-band (at 300 K). The dielectric constant is found to be high with 5 wt% f-MWCNTs as compared to the pristine P(VdF-TrFE) as shown in Fig. 12i. This high value of the dielectric constant may be due to the formation of many micro-capacitors between the MWCNTs and the polymer [58]. On incorporation of MIL (10, 20 and 30 wt%) in the polymer composite (PFE5fM), the dielectric constant further increases and is found to be maximum (~ 228) for PFE5fM20MIL composite. This enhancement in dielectric constant is attributed to large number of MILꞌs immobile anions/cations (stored charges) attached to the polymer backbone which do not contribute to the electrical conduction [63]. Consequently, the dielectric loss as well as electrical conductivity reduce as shown in Fig. 12ii & Fig. 14i.
In the polymer composites with and without MIL, dielectric losses (in high and low frequency region) are attributed to the dipolar relaxation and interfacial polarization. Also, the linear behaviour of the dielectric loss at low frequencies in the above polymer composites may be due to conduction of free electrons present at the f-MWCNTsꞌ surface [64, 65].
Figure 13i-ii show the frequency dependence of the real (µ′) and imaginary (µ″) parts of the complex permeability. The µ′ and µ″ of PFE5fM composite without MIL is less than 1 which indicates that this composite has minimal magnetic loss. On incorporation of MIL in PFE5fM, the values of µ′ further decreases while µ″ increases as shown in Fig. 13i-ii, respectively. The minimum value of permeability has been obtained for mid-composition, i.e., 20 wt% loading of MIL in the polymer composite (viz., PFE5fM20MIL). Although sufficient number of magnetic dipoles are available in this system, the microstructural changes observed (as already discussed in SEM and XRD) obstruct their alignment thereby reducing the permeability.
The effect of incorporating MIL in the PFE5fM is rise in µ′ above unity (at low frequencies) along with a slight increase in µ″ is slightly increased above zero in the entire frequency range. This magnetic loss (µ″) may be a combined effect of the motion of magnetic domains, eddy current losses and natural resonance of ferromagnetic materials [66].
As the microwave absorption by a shield material is due to the dielectric and magnetic losses being complimentary [67,68,69], the high dielectric constant and high magnetic losses of PFE5fM20MIL should lead to high microwave absorption in this composite film.
Figure 14i shows the frequency dependent electrical conductivity of the composite films at 300 K. Being an insulator, the electrical conductivity of pristine P(VDF-TrFE) obtained is ~ 10− 9-10− 10 S.cm− 1. On dispersion of 5 wt% of f-MWCNTs, the electrical conductivity increases due to the electrons present in the nanotubes. On the incorporation of 10 wt% of MIL in the PFE5fM system, the electrical conductivity further increases. This is due to the anions/cations of the MIL which make the polymer composites mixed (ion + electron) conductors. However, on addition of 20 wt% of MIL, the conductivity is found to reduce. Microstructure and porosity studies described above indicate at a porous structure with large area for this composition to which this decrement in conductivity is assigned to. The electrical conductivity (σac) has been calculated using the following relation;
As seen in this equation, the electrical conductivity depends on the frequency as well as dielectric properties of the material which are being tailored in this study.
A broad peak/hump observed at around 1.05 to 1.18 GHz in all the polymer composites (as shown in Figs. 12 and 13), indicates a resonance behaviour which is expected when the composite films are highly conducting and skin effect becomes significant [34].
Another parameter which needs to be taken into consideration while designing a microwave shield is the skin depth. The skin depth (δ) has been determined using the following equation,
Where, ω, σac, and \({\mu }^{{\prime }}\), respectively, are the angular frequency, total electrical conductivity, and relative permeability of the sample. For PFE5fM, a sample with no MIL but electronic conduction, the skin depth is very small. On addition 10 and 30 wt% of MIL to the polymer composite, the value of skin depth does not change much as the conductivity is due to both electrons and ions in these systems. The skin depth is found to be maximum (~ 18.38 μm) for PFE5fM20MIL polymer composite at 8.2 GHz frequency (as both the conductivity as well as permeability (µꞌ) decrease here). The variation in skin depth with frequency is shown in Fig. 14ii.
EMI shielding performance and mechanism
Shielding effectiveness (SE) is the ability of shielding material to shield against external EM wave and is evaluated using the equation given below;
In this equation, Pin and Pout are the power (electrical field strength) of the incident and transmitted EM waves, respectively. The overall shielding effectiveness (SET) in terms of reflection loss (SER), absorption loss (SEA), and multiple reflection loss (SEM) is given by the equation;
Multiple reflection becomes insignificant and is generally neglected when the absorption loss (SEA) is greater than 10 dB. Multiple reflection also depends on the skin depth (and hence thickness) of the shield materials. It is well known that multiple reflected waves can be absorbed by the shield material whose thickness is greater than skin depth (t > δ) [70].
Electromagnetic parameters (S-parameters) are associated with the reflectance (R = |S11|2 = |S22|2), transmittance (T = |S12|2 = |S21|2) and absorbance (A = 1-R-T). These electromagnetic parameters have been obtained experimentally using the Vector Network Analyzer in the X-band.
The total EMI shielding has been calculated adding the contributions of reflection (SER) and absorption (SEA) by using the equations;
Generally, electromagnetic (EM) radiation is reflected from conducting materials and the extent of reflection depends on the electrical conductivity and morphology of shield material whereas the absorption of EM waves can be controlled by the magnetic and dielectric properties of the shield materials. In porous polymer composites, the various interfaces provide different conducting paths to the charge carriers due to the heterogeneity in the materials. These interfaces give rise to multiple-reflections thereby improving the microwave attenuation of EM waves. These porous structures increase the path of EM wave propagation in the material contributing to high EM wave absorption [71]. With changing wt% of MIL, the pores’ size and area in the films change too which has been confirmed by BET measurement (as discussed above).
In brief, EMI shielding comprises of reflection loss generated on the surface of the shield material, absorption loss generated inside the shield material and multiple reflection loss generated between interfaces.
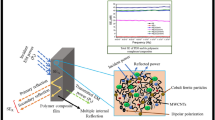
The schematic description of electromagnetic wave travelling across a porous polymer composite is shown in Fig. 15.
Figure 16i-iii represent the SER, SEA, and SET of the composite films in the X-band (8.2–12.4 GHz). In consonance with the dielectric constant, magnetic loss and conductivity observations given above, maximum shielding effectiveness had been obtained in PFE5fM20MIL (SEA ∼ 24 dB and SER ∼10 dB at a frequency of 1.23 GHz). Worth noting is the fact that the SEA values are much higher than SER values as seen in Fig. 16i-ii which was aimed at in this study, i.e., absorption was the dominant factor in the shielding mechanism. Also, due to the porous structure of the films, multiple reflection also plays an important role in enhancing the SET along with the factors mentioned above.
Figure 17 shows the conductivity and total EMI shielding effectiveness of the polymer composites with and without MIL. In PFE5fM20MIL composite film, as the pore area increases, the conductivity decreases due to connecting network being hindered. This enhanced pore area facilitates multiple reflection which ultimately enhances the SET. On the addition of 30 wt% of MIL in the polymer composite, while the pore size increases yet the conductivity also increases due to large number of anions/cations of the MIL. The decrement in the magnetic losses in this film decreases the EMI shielding capability.
Conclusion
In this paper, a shielding material with only three constituents {P(VDF-TrFE), f-MWCNTs and MIL} which is flexible, lightweight and a good microwave absorber in the X-band (8.2–12.4 GHz) has been reported. Here, the magnetic ionic liquid besides being responsible for the enhanced magnetic loss also becomes a source for ionic charge carriers (thereby reducing the need for adding another salt or ionic liquid for conductivity modification). Most studies reported on polymer composites as EMI shields have constituents more than three making their synthesis tedious and expensive. This has been taken care of by the dual role played by the magnetic ionic liquid. Also, the porosity introduced due to the incorporation of MIL in polymer composites results in increased skin depth. All these features add up to give a shield material with maximum SET ∼34 dB (PFE5fM20MIL).
References
Xu YT, Wang Y, Zhou CG, Sun WJ, Dai K, Tang JH, Lei J, Yan DX, Li ZM (2020) An electrically conductive polymer composite with a co-continuous segregated structure for enhanced mechanical performance. J Mater Chem A 8:11546–11554
Yao Y, Jin S, Zou H, Li L, Ma X, Lv G, Gao F, Lv X, Shu Q (2021) Polymer-based lightweight materials for electromagnetic interference shielding: a review. J Mater Sci 56:6549–6580
Kruželák J, Kvasničáková A, Hložeková K, Hudec I (2021) Progress in polymers and polymer composites used as efficient materials for EMI shielding. Nano Adv 3:123–172
Osawa Z, Kuwabara S (1992) Thermal stability of the shielding effectiveness of composites to electromagnetic interference. Effects of matrix polymers and surface treatment of fillers. Poly Deg Stab 35:33–43
Kim BR, Lee HK, Kim E, Lee SH (2010) Intrinsic electromagnetic radiation shielding/absorbing characteristics of polyaniline-coated transparent thin films. Syn Met 160:1838–1842
Hsiao ST, Ma CCM, Tien HW, Liao WH, Wang YS, Li SM, Huang YC (2013) Using a non-covalent modification to prepare a high electromagnetic interference shielding performance graphene nanosheet/water-borne polyurethane composite. Carbon 60:57–66
Verma M, Chauhan SS, Dhawan SK, Choudhary V (2017) Graphene nanoplatelets/carbon nanotubes/polyurethane composites as efficient shield against electromagnetic polluting radiations. Comp Part B: Eng 120:118–127
Wu J, Chung DDL (2008) Combined use of magnetic and electrically conductive fillers in a polymer matrix for electromagnetic interference shielding. J Ele Mater 37:1088–1094
Cao WQ, Wang XX, Yuan J, Wang WZ, Cao MS (2015) Temperature dependent microwave absorption of ultrathin graphene composites. J Mater Chem C 3:10017–10022
Lü Y, Wang Y, Li H, Lin Y, Jiang Z, Xie Z, Kuang Q, Zheng L (2015) MOF-derived porous Co/C nanocomposites with excellent electromagnetic wave absorption properties. ACS Appl Mater Interfaces 7:13604–13611
Kim HM, Kim K, Lee CY, Joo J, Cho SJ, Yoon HS, Pejaković DA, Yoo JW, Epstein AJ (2004) Electrical conductivity and electromagnetic interference shielding of multiwalled carbon nanotube composites containing Fe catalyst. Appl Phys Lett 84:589–591
Che RC, Peng LM, Duan XF, Chen Q, Liang AX (2004) Microwave absorption enhancement and complex permittivity and permeability of Fe encapsulated within carbon nanotubes. Adv Mater 16:401–405
Kallumottakkal M, Hussein MI, Haik Y, Abdul Latef TB (2021) Functionalized-CNT polymer composite for microwave and electromagnetic shielding. Polymers 13:3907
Gao R, Ramirez SM, Inglefield DL, Bodnar RJ, Long TE (2013) The preparation of cation-functionalized multi-wall carbon nanotube/sulfonated polyurethane composites. Carbon 54:133–142
Hsiao ST, Ma CCM, Tien HW, Liao WH, Wang YS, Li SM, Yang CY, Lin SC, Yang RB (2015) Effect of covalent modification of graphene nanosheets on the electrical property and electromagnetic interference shielding performance of a water-borne polyurethane composite. ACS Appl Mater Interfaces 7:2817–2826
Gui H, Xu P, Hu Y, Wang J, Yang X, Bahader A, Ding Y (2015) Synergistic effect of graphene and an ionic liquid containing phosphonium on the thermal stability and flame retardancy of polylactide. RSC Adv 5:27814–27822
Hu Y, Xu P, Gui H, Yang S, Ding Y (2015) Effect of graphene modified by a long alkyl chain ionic liquid on crystallization kinetics behavior of poly (vinylidene fluoride). RSC Adv 5:92418–92427
Xu P, Wang X, Hu Y, Ding Y (2016) Piezoresistive properties of nanocomposites based on silicone rubber and ionic liquid-functionalized carbon black. Mater Lett 182:218–222
Kumar R, Sahoo S, Joanni E, Singh RK, Tan WK, Kar K, Matsuda A (2021) Recent progress on carbon-based composite materials for microwave electromagnetic interference shielding. Carbon 177:304–331
Lee S, Jo I, Kang S, Jang B, Moon J, Park JB, Lee S, Rho S, Kim Y, Hong BH (2017) Smart contact lenses with graphene coating for electromagnetic interference shielding and dehydration protection. ACS Nano 11:5318–5324
Zhang QM, Bharti V, Kavarnos G (2002) Poly (vinylidene fluoride)(PVDF) and its copolymers. Encyc Smart Mater
Xia W, Zhang Z (2018) PVDF-based dielectric polymers and their applications in electronic materials. Dielectrics 1:17–31
Nalwa HS (1995) Ferroelectric polymers: chemistry: physics, and applications. CRC Press
Klein RJ, Runt J, Zhang QM (2003) Influence of crystallization conditions on the microstructure and Electromechanical Properties of Poly (vinylidene fluoride – trifluoroethylene – chlorofluoroethylene) terpolymers. Macromolecules 36:7220–7226
Lu Y, Claude J, Norena-Franco LE, Wang Q (2008) Structural dependence of phase transition and dielectric relaxation in ferroelectric poly (vinylidene fluoride – chlorotrifluoroethylene – trifluoroethylene) s. J Phys Chem B 112:10411–10416
Martins P, Lopes AC, Lanceros-Mendez S (2014) Electroactive phases of poly (vinylidene fluoride): determination, processing and applications. Progress Poly Sci 39:683–706
Wang CC, Song JF, Bao HM, Shen QD, Yang CZ (2008) Enhancement of electrical properties of ferroelectric polymers by polyaniline nanofibers with controllable conductivities. Adv Funct Mater 18:1299–1306
Santos E, Albo J, Irabien A (2014) Magnetic ionic liquids: synthesis, properties and applications. RSC Adv 4:40008–40018
Calabria L, Fernandes JA, Migowski P, Bernardi F, Baptista DL, Leal R, Grehl T, Dupont J (2017) Confined naked gold nanoparticles in ionic liquid films. Nanoscale 9:18753–18758
Joseph A, Żyła G, Thomas VI, Nair PR, Padmanabhan AS, Mathew S (2016) Paramagnetic ionic liquids for advanced applications: a review. J Mole Liquids 218:319–331
Santos E, Albo J, Irabien A (2014) Magnetic ionic liquids: synthesis, properties and applications. RSC Adv 4:40008–40018
Cruz MM, Borges RP, Godinho M, Marques CS, Langa E, Ribeiro APC, Lourenço MJV, Santos FJV, de Castro CN, Macatrão M, Tariq M, Rebelo LPN (2013) Thermophysical and magnetic studies of two paramagnetic liquid salts:[C4mim][FeCl4] and [P66614][FeCl4]. Flui Phase Equi 350:43–50
Ribeiro C, Costa CM, Correia DM, Nunes-Pereira J, Oliveira J, Martins P, Gonçalves R, Cardoso VF, Lanceros-Mendez S (2018) Electroactive poly (vinylidene fluoride)-based structures for advanced applications. Nat Protoc 13:681–704
Kowsari E, Mohammadi M (2016) Synthesis of reduced and functional graphene oxide with magnetic ionic liquid and its application as an electromagnetic-absorbing coating. Comp Sci Tech 126:106–114
Sanoja-López KA, Quiroz-Suárez KA, Dueñas-Rivadeneira AA, Maddela NR, Montenegro MC, Luque R, Rodríguez-Díaz JM (2022) Polymeric membranes functionalized with nanomaterials (MP@ NMs): A review of advances in pesticide removal. Environ Res 114776
Wang C, Murugadoss V, Kong J, He Z, Mai X, Shao Q, Chen Y, Guo L, Liu C, Angaiah S, Guo Z (2018) Overview of carbon nanostructures and nanocomposites for electromagnetic wave shielding. Carbon 140:696–733
Sankaran S, Deshmukh K, Ahamed MB, Pasha SK (2018) Recent advances in electromagnetic interference shielding properties of metal and carbon filler reinforced flexible polymer composites: a review. Comp Part A: Appl Sci Manu 114:49–71
Farbod M, Tadavani SK, Kiasat A (2011) Surface oxidation and effect of electric field on dispersion and colloids stability of multiwalled carbon nanotubes. Coll Surf A: Physico Eng Asp 384:685–690
Dandekar A, Baker RTK, Vannice MA (1998) Characterization of activated carbon, graphitized carbon fibers and synthetic diamond powder using TPD and DRIFTS. Carbon 36:1821–1831
do Amaral Montanheiro TL, Cristóvan FH, Machado JPB, Tada DB, Durán N, Lemes AP (2015) Effect of MWCNT functionalization on thermal and electrical properties of PHBV/MWCNT nanocomposites. J Mater Research 30:55–65
Rath SK, Dubey S, Kumar GS, Kumar S, Patra AK, Bahadur J, Singh AK, Harikrishnan G, Patro TU (2014) Multi-walled CNT-induced phase behaviour of poly (vinylidene fluoride) and its electro-mechanical properties. J Mater Sci 49:103–113
Jorio A, Dresselhaus G, Dresselhaus MS, Souza M, Dantas MSS, Pimenta MA, Rao AM, Saito R, Liu C, Cheng HM (2000) Polarized Raman study of single-wall semiconducting carbon nanotubes. Phys Rev Lett 85:2617
Datsyuk V, Kalyva M, Papagelis K, Parthenios J, Tasis D, Siokou A, Kallitsis I, Galiotis C (2008) Chemical oxidation of multiwalled carbon nanotubes. Carbon 46:833–840
Dresselhaus MS, Dresselhaus G, Jorio A, Souza Filho AG, Saito R (2002) Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 40:2043–2061
Scheibe B, Borowiak-Palen E, Kalenczuk RJ (2010) Oxidation and reduction of multiwalled carbon nanotubes—preparation and characterization. Mater Character 61:185–191
Murphy H, Papakonstantinou P, Okpalugo TT (2006) Raman study of multiwalled carbon nanotubes functionalized with oxygen groups. J Vac Sci Tech B: Micro Nano Stru Proce Measu Pheno 24:715–720
Wang F, Kong Y, Liu Z, Zhu H (2017) All-organic poly (butyl methacrylate)/Poly (vinylidenefluoride-trifluoroethylene) dielectric composites with higher permittivity and low dielectric loss for Energy Storage Application. Poly Plas Tech Eng 56:526–534
Durak Ö, Zeeshan M, Habib N, Gülbalkan HC, Alsuhile AA, Çağlayan HP, Kurtoğlu-Öztulum SF, Zhao Y, Haşlak ZP, Uzun A, Keskin S (2022) Composites of porous materials with ionic liquids: synthesis, characterization, applications, and beyond. Microporous Mesoporous Mater 332:111703
Yoshida Y, Otsuka A, Saito G, Natsume S, Nishibori E, Takata M, Sakata M, Takahashi M, Yoko T (2005) Conducting and magnetic properties of 1-ethyl-3-methylimidazolium (EMI) salts containing paramagnetic irons: liquids [EMI][MIIICl4](M = fe and Fe0. 5Ga0. 5) and solid [EMI]2 [FeIICl4]. Bulle Chem Soci Japan 78:1921–1928
Xie ZL, Jeličić A, Wang FP, Rabu P, Friedrich A, Beuermann S, Taubert A (2010) Transparent, flexible, and paramagnetic ionogels based on PMMA and the iron-based ionic liquid 1-butyl-3-methylimidazolium tetrachloroferrate (III)[Bmim][FeCl4]. J Mater Chem 20:9543–9549
Mayeen A, Kala MS, Jayalakshmy MS, Thomas S, Philip J, Rouxel D, Bhowmik RN, Kalarikkal N (2019) Flexible and self-standing nickel ferrite–PVDF-TrFE cast films: promising candidates for high-end magnetoelectric applications. Dalt Trans 48:16961–16973
Prasanna CM, Suthanthiraraj SA (2016) Effective influences of 1-ethyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide (EMIMTFSI) ionic liquid on the ion transport properties of micro-porous zinc-ion conducting poly (vinyl chloride)/poly (ethyl methacrylate) blend-based polymer electrolytes. J Poly Research 23:1–17
Skakalova V, Kaiser AB, Dettlaff-Weglikowska U, Hrncarikova K, Roth S (2005) Effect of chemical treatment on electrical conductivity, infrared absorption, and Raman spectra of single-walled carbon nanotubes. J Phy Chem B 109:7174–7181
Dresselhaus MS, Jorio A, Hofmann M, Dresselhaus G, Saito R (2010) Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett 10:751–758
Wang Y, Iqbal Z, Mitra S (2006) Rapidly functionalized, water-dispersed carbon nanotubes at high concentration. J Ame Chem Soci 128:95–99
Zhang D, Dong QQ, Wang X, Yan W, Deng W, Shi LY (2013) Preparation of a three-dimensional ordered macroporous carbon nanotube/polypyrrole composite for supercapacitors and diffusion modeling. J Phy Chem C 117:20446–20455
Liu H, Zhao R, Song X, Liu F, Yu S, Liu S, Ge X (2017) Lewis acidic ionic liquid [Bmim] FeCl4 as a high efficient catalyst for methanolysis of poly (lactic acid). Catal Lett 147:2298–2305
Vyas MK, Chandra A (2016) Ion–electron-conducting polymer composites: promising electromagnetic interference shielding material. ACS Appl Mater Interfaces 8:18450–18461
Chang CM, Liu YL (2011) Electrical conductivity enhancement of polymer/multiwalled carbon nanotube (MWCNT) composites by thermally-induced defunctionalization of MWCNTs. ACS Appl Mater Interfaces 3:2204–2208
De Volder MFL, Tawfick SH, Baughman RH, Hart AJ (2013) Carbon nanotubes: present and future commercial applications. Science 339:535–539
Fu J, Li Z, Zhou X, Guo X (2022) Ion transport in composite polymer electrolytes. Mater Adv 3:3809–3819
Döbbelin M, Jovanovski V, Llarena I, Marfil LJC, Cabañero G, Rodriguez J, Mecerreyes D (2011) Synthesis of paramagnetic polymers using ionic liquid chemistry. Poly Chem 2:1275–1278
Buraidah MH, Teo LP, Majid SR, Arof AK (2009) Ionic conductivity by correlated barrier hopping in NH4I doped chitosan solid electrolyte. Phy B: Cond Matt 404:1373–1379
Pötschke P, Dudkin SM, Alig I (2003) Dielectric spectroscopy on melt processed polycarbonate-multiwalled carbon nanotube composites. Polymer 44:5023–5030
Dakin TW (2006) Conduction and polarization mechanisms and trends in dielectric. IEEE Ele Insul Mag 22:11–28
Che RC, Peng LM, Duan XF, Chen Q, Liang AX (2004) Microwave absorption enhancement and complex permittivity and permeability of Fe encapsulated within carbon nanotubes. Adv Mater 16:401–405
Fan Z, Luo G, Zhang Z, Zhou L, Wei F (2006) Electromagnetic and microwave absorbing properties of multi-walled carbon nanotubes/polymer composites. Mater Sci Eng B 132:85–89
Li G, Hu GG, Zhou HD, Fan XJ, Li XG (2002) Attractive microwave-absorbing properties of La1-xSrxMnO3 manganite powders. Mater Chem Phy 75:101–104
Wei J, Liu J, Li S (2007) Electromagnetic and microwave absorption properties of Fe3O4 magnetic films plated on hollow glass spheres. J Magne Magne Mater 312:414–417
Tan YJ, Li J, Cai JH, Tang XH, Liu JH, Hu ZQ, Wang M (2019) Comparative study on solid and hollow glass microspheres for enhanced electromagnetic interference shielding in polydimethylsiloxane/multi-walled carbon nanotube composites. Comp Part B: Eng 177:107378
Singh AK, Shishkin A, Koppel T, Gupta N (2018) A review of porous lightweight composite materials for electromagnetic interference shielding. Comp Part B: Eng 149:188–197
Acknowledgements
Financial support from University of Delhi, Institution of Eminence (Ref. No./IoE/2021/12/FRP) and the Alexander von Humboldt Foundation (through its Research Group Linkage programme) is gratefully acknowledged. Also, thanks to CSIR, New Delhi, India for providing the financial support (09/045(1600)/2018-EMR-1) to one the authors (Anjana). Prof. S. A. Hashmi, Department of Physics & Astrophysics, Mr. Ravinder Singh and Mr. Govind Singh, Department of Chemistry University of Delhi for providing BET, XRD and TGA facilities are gratefully acknowledged. Authors also thank the University Science Instrumentation Centre (USIC), University of Delhi for providing the characterization facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that there are no known competing economic benefits that may affect the studies reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Anjana, Chandra, A. Dual role of magnetic ionic liquid in EMI absorbing polymer composites of P(VDF-TrFE) and f-MWCNTs. J Polym Res 30, 78 (2023). https://doi.org/10.1007/s10965-023-03460-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03460-9