Abstract
Waterborne polyurethane (WPU) with different macromolecular diols as soft segments was synthesized by the acetone dilution method. Fourier transform infrared (FTIR) spectroscopy, particle size and distribution (PSD) and transmission electron microscopy (TEM) test measurements were utilized to characterize the structure. The effect of the structure on performance was determined by the solids content, rheological and thixotropic behavior tests, water absorption tests and adhesion tests. It was found that with different macromolecular diols as a soft segment polyurethane has low water absorption, high energy storage, good shear resistance and adhesion. The minimum water absorption value was 6.02%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, waterborne polyurethane has become one of the major research and development fields in recent years because of its environmental friendliness. Meanwhile waterborne polyurethane material has attracted more and more attention. Polyurethane (PU) is widely used in industries as soft coatings for textiles or leathers and as hard coatings for wood because their perceived quality as well as physical properties, such as flexibility, adhesion and abrasion resistance, are superior compared with other polymeric materials [1,2,3]. In addition, a tailor-made chemical structure, and thus the desired property, can be designed appropriately [4,5,6]. Among them Haihua Wang, qili et al. modified the polyurethane by introducing different functional groups [7]. For polyurethanes, the properties of the soft segment have a great influence on the system, so it is very necessary to study the composition of the soft segment. Taeyi Choi et al. studied the effect of soft segment changes on the morphology, phase change and phase separation of microphase separation systems by changing the content of soft segments [8,9,10,11,12,13]. Different kinds of macromolecule diols have a great influence on the soft segment of the polyurethane. Poly-1, 4-butylene adipate glycol (PBA), Polycaprolactone diol (PCL) and Poly (propylene glycol) (PPG) are biodegradable aliphatic material with excellent tensile property and are extensively used as polyol in polyurethane industry. Meng-Shung YenShu-Chin Kuo et al. synthesized waterborne polyurethanes with good mechanical properties by introducing PEG into PCL-based soft segments [14]. Numerous studies have confirmed that Many studies have confirmed that the type of macrodiol has a great influence on the properties of polyurethanes. The physical and chemical properties of polyurethane can be changed by using different kinds of macromolecular glycols [15,16,17,18,19,20,21,22,23].
In this paper a new series of polyurethanes were synthesized by use of two different kinds of macromolecular glycols. Some properties of these polyurethanes were studied.
Experimental section
Materials
Isophorone diisocyanate (IPDI, 99%) and N-methyl-2-pyrrolidone (NMP, AR) were supplied by Shangdong Xiya Chemical Industry Co. Ltd. PBA (Mn = 2000), PEG, (Mn = 400), supplied by Chengdu Huaxia reagent Corporation, were dried by vacuum drying oven at 90 °C for 16 h. PCL (Mn = 2000), supplied by Sigma-Aldrich Chemistry, were dried by vacuum drying oven at 90 °C for 16 h; PPG (Mn = 2000), supplied by J&K Scientific Ltd. Dimethylol propionic acid (DMPA, 99%) was purchased from USA New Jersey. Dibutyltin didodecylate (DBTDL) was supplied by Sinopharm Chemical Regent Co. Ltd. 2-Hydroxyethyl acrylate (HEA, 97%) was supplied by Shanghai Macklin biochemical Co. Ltd. 3-Aminopropyl-trithpxysilane (KH550, 99%) was supplied by Acros Organics. 1,4-butanediol (BDO, AR, 99%) and triethyl amine (TEA, AR, 99%) were receieved from Tianjin Guangfu Fine Chemical Research Institute. Deionized water was used for the whole work and all other materials were used without further purification. Poly (vinyl chloride) (PVC), biaxially oriented polypropylene (OPP), polyamide (PA), polyethylene (PE) and poly (ethylene terephthalate) (PET) were purchased from the market.
Synthesis of waterborne polyurethanes with different macrodiols as soft segments
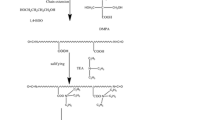
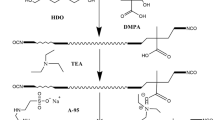
The synthesis of the PU was carried out in a 250 mL four-necked glass reactor equipped with a thermometer and a mechanical stirrer. The synthesis was performed under nitrogen atmosphere. First, Macrodiols, IPDI and DBTDL were poured into the reactor at 80 °C. After that, when the temperature was cooled to 60 °C, acetone was added for decreasing the viscosity of PU prepolymer, and then DPMA was added to the container to react for 3 h. Then BDO was added into the system for 1 h. After that, HEA and KH550 were added into the system and the temperature remained constant for another hour at 70 °C. When the reaction mixture was cooled down to 40 °C, TEA was added into the reactor for 0.5 h. Then deionized water was added into the flask under the mechanical stirring with the stirring rate of 550 rpm for 1 h to obtain polyurethane dispersion (PUD). The whole synthesis process of WPUs are shown in Scheme 1, and the recipe of WPUs are listed in Table 1.
Preparation of WPUs films
The films were obtained by pouring the WPU dispersions onto a glass molds to dry at room temperature for 7 day and then at 50 °C in a vacuum dry oven for 24 h. Then the films were stored into desiccator for further study.
Characterizations
FTIR spectral analysis
The infrared spectra measurements of samples were recorded on the Nicolet 5700 instrument (Thermo Company, USA). All of the spectra were scanned within the range 600–4000 cm−1.
Particle size and distribution (PSD)
The particle size and distribution of the WPU dispersions were analyzed by the Malvern Zetasizer Nano-ZS instrument under 25 °C. The sample was first diluted in deionized water to a concentration of 0.4 wt%, and then was treated by ultrasonic wave to homogenize the dispersion.
Transmission electron microscopy (TEM) test
Transmission electron microscopy (TEM) images of the WPU dispersions were obtained from a JEM-2100 Microscope with an accelerating voltage of 120 kV. Before observation, the samples were stained by 2 wt% phosphor tungstic acid hydrate, which was adjusted exactly to pH of 7 with 0.1 mol/L NaOH aqueous solution.
Solid content
The solid content of the WPU dispersions were detected by drying some emulsion (about 2.50 g) at 55 °C for more than 24 h and then calculating the weight ratio of residue to the whole mass.
Rheological and thixotropy behavior test
The shear viscosity and thixotropy of emulsions were determined by Thermo Scientific HAKE MARS (CC25 DIN Ti) at 25 °C. The shear rate ranged from 1 s−1 to 2000 s−1 and then reduced from 2000 s−1 to 1 s−1.
Water absorption test
Water absorption of the cast films was assessed according to the standard method. Dry films cast from emulsion (original weight designated as w1) were immersed in water for 24 h at 25 °C. After the residual water was wiped from the films using filter paper, the weight (w2) was measured immediately. Water absorption was calculated by the equation, W (%) = (w2− w1)/w1× 100%.
Adhesion test
The adhesion tests on packaging films (PVC, OPP, PA, PE, and PET) were tested according to ASTM standards D 3359–09. A lattice pattern with eleven cuts in each direction was made in the emulsion film to the substrate, pressure-sensitive tape was applied over the lattice and then removed, and adhesion was evaluated by comparison with descriptions and illustrations. Table 2 shows the classification for assessment of the damaged films surface, i.e. the adhesion of the films to the substrate.
Results and discussion
FTIR spectra of WPUs
Figure 1a shows the FTIR spectra of PU550, PU505, PU055, PU1000, PU0100, PU0010 and Fig. 1b shows the FTIR spectra of IPDI. It is found that the characteristic peak at around 1684 cm−1 for the carbonyl of -COOH disappeared, and the strong vibration absorption peak at 1727 cm−1 for carbonyl of ester emerged. The fact indicates the introduction of butyl acrylate monomer. The bands area in the range of 1100 cm−1 and 1108 cm−1 belong to the stretching vibration of C-O-C, and the wide characteristic absorption peak at 3325 cm−1 is assigned to the stretching vibration of saturated N-H. There is no characteristic peak at 2247 cm−1 in Fig. 1a, which proves that -NCO in the synthesized PU dispersion has completely reacted. The 1727 cm−1 (C=O) and 3325 cm−1 (N-H) peaks appear in Fig. 1b, demonstrating that -NCO and -OH have been successfully combined to form a urethane bond (-CO-NH-).
Solid content, particle size, potential and TEM of WPUs
The size of WPUs are mainly controlled by the type and content of macrodiols. The backbone of polymer with rich hydrophilic units (–COO−) is easy to be dispersed by water [24]. The whole dispersion achieves phase inversion from water in oil (W/O) to oil in water (O/W) with an electrical double layer structure obtained. The particle size test (Fig. 2) showes that all PUDs give a monomodal distribution. In order to prove the polyurethane particle size results, TEM was measured. The TEM photographs (Fig. 4) agreed with the schematic diagram of the particle size data well as shown in Figs. 3 and 4, which prove that WPU with monomodal PSD is successfully synthesized [24].
From the results of the particle size as shown in Fig. 3, the introduction of PPG will make the PU particle size smaller, and the introduction of PBA and PCL will make the PU part of the particle size significantly increase, on the one hand because the structure of PBA and PCL are harder than PPG. On the other hand, PBA and PCL have more hydrophobic groups (ester groups), which will lead to phase inversion in water dispersion process more difficult to carry out. Therefore, in order to maintain the stability of O/W, it will need a larger amount of hydrophilic groups on the particle surface flip. This is a direct result of increased particle size. In this case, the PCL segment is softer than PBA. Under the same conditions, the PU particle size of PCL is smaller than that of PBA.
Table 3 shows the potential of WPUs. As Table 3 shows, The absolute value of the potential of the stable system was greater than 31 mV. Since the content of DMPA was fixed, the influence of the double electron layer was basically the same, and the main cause of the difference in particle size was the different composition of the soft segment.
Potential absolute value of all samples is larger than 40 mV, indicating that PUs have good storage stability at 25 oC.
Rheological and thixotropy behavior test
Figure 5 described the rheological behavior of WPUs, which mainly involved in the relationship between shear viscosity and shear rate at 25 °C. In Fig. 5, the shear viscosity of most of WPUs are reduced with the increase of shear rate along with a parabolic recession. This result reveals that these WPUs exhibit the pseudoplastic liquid behaviors. This was because the cohesive energy (12.2 kJ/m−1) of the ester in the polyester was much larger than the cohesive energy (4.2 kJ/m−1) of the ether group in the polyether, which made the interaction stronger between the molecules of the polyurethane. At the same time, because the polarity of the ester group was large, the cohesive energy was high, the internal rotation was difficult, and the ether bond was linear, and the inner rotation was relatively easy. Therefore, the addition of a soft segment containing an ether bond in a polyester-type polyurethane increased the shear resistance of the polyurethane and caused the sample to exhibit a pseudoplastic liquid behavior. Exceptions are PU1000 and PU0100. These samples approximate newtonian fluid at the experiment shear rates. The shear rate ranged from 1 s−1 to 2000 s−1 and then reduced from 2000 s−1 to 1 s−1. The initial viscosity of All samples exhibited good shear resistance by increasing the shear rate from a low shear rate (10 s−1) to a high shear rate (2000 s-1) and then back to a low shear rate (10s-1). During the process of all sample, the shear viscosity of the sample can be returned to near or close to the initial value, which proves that the synthesized sample has a certain shear stability and can be applied to the preparation of aqueous ink.
Thixotropy behavior measurement was used to investigate the storage modulus and loss modulus of WPUs with different macrodiols contents. Figure 6 shows the storage modulus of WPUs. The storage modulus (G’) is found to increase after the increase of content of PBA, indicating that the incorporation of PBA or PCL improves the stiffness of WPU. From the perspective of molecular structure, there are more ester groups in the molecular chains of PBA and PCL, which makes the PU segment harder. Such a structure leads to a larger storage modulus of the synthesized WPU.
All PUs show little difference in storage modulus values at low frequencies, and as the frequency increases, the addition of a more structurally stiff diol causes the PU loss modulus to be greater than the storage modulus, which means that the addition of more PBA or PCL tends to make PUs tend to stick; otherwise adding more PPG tends to make WPUs tend to elastomer.
Water absorption of WPU films
From the analysis of water absorption results (Fig. 7), the introduction of PBA and PCL make the WPUs dry films water absorption significantly reduced, due to the introduction of a large number of hydrophobic groups in the chain. Because there is more -CH2 between two adjacent ester groups in PCL, which lead to a better hydrophobic effect of PCL. Therefore, introducing the hydrophobic PCL decreased effect more pronounced than PBA.
According to the data of water absorption in Fig. 7, PU550 gets the minimum water absorption value (6.02%) among them.
Adhesion of WPUs
The adhesion tests of the WPUs on packaging films (PVC, BOPP, PA, PE and PET) were taken by a cross-cut tape test. The results are shown in Table 5. As is seen in Table 5, all samples on PVC, PA, PE, PET films showed excellent adhesion behaviors. The adhesion of PU550 on BOPP film gets 2B. The reason for the results can be ascribed to two factors. First, BOPP films belong to non-polar substrates with low surface free energy. Second, BOPP was a biaxially stretched polypropylene film. Therefore, the WPU could hardly stick to the surface of this film.
The surface energy of the substrate was available in references. Surface energy data has already listed in Tables 4 and 5 as follows:
Thermal stability
WPUs films were obtained by casting the waterborne polyurethane into a Teflon mold and allowing it to dry out at ambient temperature. Thermal stability of WPU films were studied using TG (Fig. 8) and DTG (Fig. 9), and the results were summarized in Table 6. T10 and T50 represent the temperature at which WPUs films lose weight at 10% and 50%, respectively. The thermogram of WPUs shows three stages of thermalde composition in N2 atmosphere. In the first stage, the degradation is attributed to urethane linkage decomposition, which results in the formation of isocyanate, primary or secondary amine and olefin, and carbon dioxide. The second stages corresponds to decomposition of soft segment (chain scission of polyol) and the third stage, above 380 °C, is attributed to further oxidation of the WPUs in air. There is no obvious trend in the range of 220–380 °C, because the structure of PBA, PCL or PPG has not changed dramatically. In addition, the DTA results confirm three degradation stages in TGA. As is shown in Fig. 9, the first, second and third decomposition stages are 250–300 °C, 300–400 °C and 400–500 °C, respectively. WPUs with PPG get higher T50 temperature. T50 is the second decomposition peak temperature, where the soft segments (mostly macrodiols) thermally decompose and the PPG segments predominately alkanes and ether linkages are still more stable than ester linkages of PBA and PCL, which leads to stronger thermal stability of the WPU.
Conclusions
Polyurethane with different components was successfully synthesized by acetone dilution method. The experimental results indicate that the soft segment composed of different macrodiols has a significant influence on the properties of polyurethane. The results also show that all the samples show good shear resistance. The enhancement in storage stability and thermal stability of emulsions is attributed to the change of macrodiols. The lower the rigidity of the macrodiol, the better the thermal stability is obtained. The addition of different macromolecular diols to the soft segments improves the performance of the PU, achieving good adhesion and low water absorption. In general, it was observed that the polyurethane with different macromolecule diol is better than the traditional polyurethane. Thus the components of the soft segment has a great impact on the performance of WPUs.
References
Doyle EN (1971) The development and use of polyurethane products. McGraw-Hill
Wang H, Zhou Y, He M, Dai Z (2015) Effects of soft segments on the waterproof of anionic waterborne polyurethane. Colloid Polym Sci 293:875–881
Lu Y, Larock RC (2007). Biomacromolecules. 8:3109
Gurunathan T, Mohanty S, Nayak SK (2014). J Mater Sci 49:8017
Chang WH, Scriven RL, Peffer JR, Jr SP (1973). Ind Eng Chem Prod Res Develop 12:282
Wang H, Niu Y, Fei G, Shen Y, Lan J (2016) In-situ polymerization, rheology, morphology and properties of stable alkoxysilane-functionalized poly (urethane-acrylate) microemulsion[J]. Progress in Organic Coatings 99:400–411
Li Q, Guo L, Qiu T, Xiao W, du D, Li X (2016) Synthesis of waterborne polyurethane containing alkoxysilane side groups and the properties of the hybrid coating films[J]. Appl Surf Sci 377:66–74
Guyot A, Landfester K, Schork FJ et al (2007) Hybrid polymer latexes[J]. Prog Polym Sci 32(12):1439–1461
Hirose M, Zhou J, Nagai K (2000) The structure and properties of acrylic-polyurethane hybrid emulsions[J]. Progress in Organic Coatings 38(1):27–34
Patil CK, Rajput SD, Marathe RJ, Kulkarni RD, Phadnis H, Sohn D, Mahulikar PP, Gite VV (2017) Synthesis of bio-based polyurethane coatings from vegetable oil and dicarboxylic acids[J]. Progress in Organic Coatings 106:87–95
Zhang SF, Wang RM, He YF, Song PF, Wu ZM (2013) Waterborne polyurethane-acrylic copolymers crosslinked core–shell nanoparticles for humidity-sensitive coatings[J]. Progress in Organic Coatings 76(4):729–735
Lu Y, Hao J, Xiao G, Chen L, Wang T, Hu Z (2017) Preparation and properties of in situ amino-functionalized graphene oxide/polyimide composite films[J]. Appl Surf Sci 422:710–719
Wang L, Zhu Y, Qu J (2017) Preparation and assistant-film-forming performance of aqueous polyurethane dispersions[J]. Progress in Organic Coatings 105:9–17
Yen MS, Kuo SC (1998) Effects of a soft segment component on the physical properties of synthesized waterborne polyurethanes by using triblock ester-ether copolydiol as the soft segment. J Polym Res 5:125–131. https://doi.org/10.1007/s10965-006-0048-5
Wang Y, Lue A, Zhang L (2009) Rheological behavior of waterborne polyurethane/starch aqueous dispersions during cure[J]. Polymer 50(23):5474–5481
Peng SJ, Jin Y, Cheng XF, Sun TB, Qi R, Fan BZ (2015) A new method to synthesize high solid content waterborne polyurethanes by strict control of bimodal particle size distribution[J]. Progress in Organic Coatings 86:1–10
Yuan C, Wang J, Cui M et al (2016) Aqueous PUA emulsion prepared by dispersing polyurethane prepolymer in polyacrylate emulsion[J]. Journal of Applied Polymer Science 133(11) n/a-n/a.17
Koscielecka A (2010) Thermal stability of polyurethanes with allophanate and isocyanurate crosslinks[J]. Acta Polymerica 42(5):221–225
Zhang J, Li X, Shi X, Hua M, Zhou X, Wang X (2012) Synthesis of core-shell acrylic-polyurethane hybrid latex as binder of aqueous pigment inks for digital inkjet printing[J]. Progress in Natural Science:Materials International 22(1):71–78
Wang C, Chu F, Guyot A (2006) Mechanical properties of films from hybrid acrylic-polyurethane polymer colloids[J]. Journal of Dispersion Science & Technology 27(3):325–330
Qiu FX, Zhang JL, Wu DM et al (2013) Waterborne polyurethane and modified polyurethane acrylate composites[J]. Plastics Rubber & Composites 39(10):454–459
Sheng Y, Jiang P, Zhang D, Hua J (2015) Synthesis and characterization of sustainable polyurethane modified by cyclic polysiloxane[J]. J Appl Polym Sci 132(2)
Teramoto N, Saitoh Y, Takahashi A, Shibata M (2010) Biodegradable polyurethane elastomers prepared from isocyanate-terminated poly(ethylene adipate), castor oil, and glycerol[J]. J Appl Polym Sci 115(6):3199–3204
Jinwei H, Duoxian S, Zhenyu Y (2003) Study on particle size and viscosity properties of waterborne polyurethane[J]. Journal of Tianjin University: Natural Science and Engineering Technology 36(6):719–723
Acknowledgements
This work is supported by the National Key R&D Program of China (2017YFB0308700), The National Natural Science Foundation of China (21676003), Beijing Municipal Science and Technology Project (D17110500190000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, N., Xin, X., Liu, H. et al. Effects of different macrodiols as soft segments on properties of waterborne polyurethane. J Polym Res 26, 152 (2019). https://doi.org/10.1007/s10965-019-1793-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1793-6