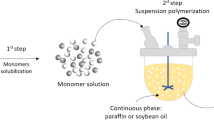
Abstract
Various heterogeneous polymerization techniques such as suspension, emulsion, dispersion, precipitation and seeded are employed for the synthesis of a wide variety of porous polymer particles. In present review, suspension polymerization technique is highlighted in detail with control of particle size, advantages and its applications. The aim of the review is to understand the basics of suspension polymerization for the synthesis of polystyrene cross-linked with divinylbenzene copolymer. Also, the effect of various synthesis parameters (agitation speed, temperature, initiator, cross-linker and diluent) on particle surface morphology, particle size and distribution, surface area and cross-linking density was reviewed and their application as catalyst support for various oxidation reactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The wide range of applications of porous polymer particles in various fields such as biotechnology, column packing for gel permeation chromatography and as a catalyst support describe their immense significance to the researchers. Based on the porosity, polymer particles are distinguished as gel and porous. Gel particles are non-porous and low cross-linked, whereas porous polymer particles, especially the ones that are spherical in shape, are highly cross-linked in nature. They have been categorized into three classes, microporous (<2 nm), mesoporous (2–50 nm) and macroporous (>50 nm), depending on their pore size (Fig. 1). These differences of porous particles from gel type particles give rise to exceptional important features such as high surface area, ability to uptake various solvents and high brittleness [1,2,3,4].
Several methods for the production of polymer particles by various heterogeneous polymerization techniques exist in which the initial monomer and resulting polymer are exist in form of a fine dispersion [5, 6]. The polymerization initiator may/may not be present within the formed polymer during their synthesis and may be soluble in monomer/medium, depending upon the type of polymerization technique. Monomer phase referred as dispersed phase/organic phase and the liquid phase comprising the dispersed phase is referred to as polymerization medium. There are basically five different techniques employed for the synthesis of polymer particles such as suspension, emulsion, dispersion, precipitation and seeded polymerization.
Suspension polymerization technique is usually suitable for the synthesis of large polymer particles (5–1000 μm), while the other processes produce much smaller particles (Fig. 2). In all cases, the application should be kept in mind prior to choosing the method of production [7,8,9,10,11,12,13,14,15,16,17]. In this technique, both initiator and monomer are insoluble in the polymerization medium, while initiator is soluble in the monomer and polymerization takes place in the monomer droplets. The monomer phase is suspended in the medium in the form of small droplets by means of a stirrer and a suitable suspension agent. Examples of polymers synthesized via suspension polymerization include PS, PVC, PAc, PVA, PAAm and water-soluble acrylates [18, 19].
In emulsion polymerization, monomer is insoluble in the polymerization medium which is emulsified by the addition of a surfactant/emulsifier/soap whereas initiator is soluble in the medium and not in the monomer. Under these conditions, the monomer is present in the mixture partly in the form of droplets and soap-coated micelles, depending on the nature and concentration of the emulsifier. The ratio of monomer phase to medium is generally 0.1–0.5. Examples of polymers produced by emulsion polymerization include PS, PMMA and PAAm, etc. [20,21,22,23,24,25].
In dispersion polymerization, polymerization is originated in homogeneous solution as both monomer and initiator are soluble in the polymerization medium. Depending on the solvency of the solution, phase separation occurs at an early stage, which leads to formation of primary particles, thus swollen by the polymerization medium. Therefore, polymerization reaction proceeds largely within the distinct droplets, leading to the formation of spherical shaped polymer particles. Examples of polymers formed via dispersion polymerization include PS, PMMA, etc. [26,27,28,29,30].
Precipitation polymerization is a non-homogeneous technique that starts with homogeneous system where both initiator and monomer are completely soluble but formed polymer is insoluble and therefore precipitates. Examples of polymers synthesized through precipitation polymerization include TFE, ACN, etc. [31,32,33,34,35,36].
In seeded polymerization technique, monodisperse seed particles formed by suspension/emulsion polymerization are treated first with a suitable oligomer, and then with monomer and an oil-soluble initiator. This mixture is completely absorbed by the particles and successive polymerization leads to the formation of larger monodisperse particles. This is an excellent technique where monodisperse particles can be grown from few micrometers to hundreds of micrometer by consecutive monomer swelling. Examples of polymers produced by seeded polymerization include PS, PMMA, etc. [37,38,39].
Seeded polymerization method is costly and complex to be carried out, while dispersion and precipitation techniques are comparatively easy and efficient for the synthesis of monodisperse polymer particles with one drawback that organic solvent is unfriendly to the environment. The difference between suspension and emulsion polymerization lies in their particle size. Emulsion technique contains particles less than 1 μm in size whereas suspension contains greater than 1 μm. Table 1 shows the comparison of different polymerization techniques on the basis of different parameters. Hence, one should consider several parameters (size, size dispersion, pore size, porosity, shape, and cost) before choosing the polymerization method for the synthesis of polymer particles [40, 41].
Based on the literature review, suspension polymerization method is simple to operate and easy to perform. However, its application is limited due to large particle size, size distribution and high value of coefficient of variation (CV; ratio of standard deviation to the mean of particle size). Higher the value of CV, higher the level of dispersion in particle size, which is usually not required. Although suspension polymerization method is not a new technology, but, if small size monodisperse-porous polymer particles could be produced from this technique by altering various synthesis parameters (e.g. amount and type of initiator, crosslinker and diluent, agitation speed and temperature), then it can be a cost-effective technique which has the ability to scale up in industry [9, 42,43,44,45,46,47,48,49,50,51,52]. Thus, the current section comprises the literature survey of suspension polymerization method in brief.
History of suspension polymerization
This technique was first developed by Hoffman and Delbruch in 1909 [53]. Around 20–30 years later, Svec et al. studied the synthesis of porous polymer particles using free radical suspension polymerization [54,55,56,57,58,59,60,61]. The internal particle morphology in suspension polymerization was first predicted by A. H. Alexopoulos and co-workers [62] and L. Tan have reviewed the synthesis of hyper cross-linked porous polymers [63]. Although suspension polymerization has been broadly studied over more than 50 years, but the present situation is that its understanding is still limited and lack of its application at the industrial level. Also, effect of various parameters on polymer synthesized via suspension polymerization are not considered in detail in previous reviews, therefore, the aim of the present review is to study the effect of various synthesis parameters for the production of PS-co-DVB copolymers.
Figure 3 shows the schematic flow diagram for the development of suspension polymerization technique.
Suspension polymerization
Outline
Suspension means the solid/liquid dispersion. It starts with liquid/liquid dispersion and ends with solid/liquid dispersion. The least sophisticated heterogeneous polymerization technique starts with the formation of organic and aqueous phases separately. Organic phase to aqueous phase volume ratio usually varies from 0.1 to 0.5 or even more. The organic phase is prepared by the addition of monomer, initiator, crosslinker molecules and diluents, on the other hand, surfactant and stabilizer are dissolved in distilled water to make aqueous phase [77,78,79,80]. Monomer droplets are dispersed in an aqueous phase with the addition of surfactant molecules (sodium dodecyl sulfate/sodium sulfate) and stabilizer (methylcellulose/PVA/gelatin) to prevent coalescence and breakage during polymerization. Also, monomer soluble initiator (free radical) is added for both initiation and chain growth mechanism within the monomer droplets, however monomer and initiator, both are insoluble in the medium. The initiation and propagation mechanism occurs inside the monomer droplets. The product is collected by conventional filtration and washed to eliminate stabilizer and other contaminants [81,82,83].
The terms pearl and bead polymerization are also used for suspension polymerization when the porosity of polymer particles is not desired. Suspension polymerization may be classified into two types on the basis of polymer solubility in the monomer i.e. suspension bead and suspension powder polymerization (Fig. 4). An example of PS-co-DVB copolymer synthesis is presented to understand the synthesis mechanism of suspension polymerization, followed by the study of effect of various synthesis parameters.
Synthesis of PS-co-DVB copolymer
PS is one of the most widely used polymer due of its salient features such as low cost, readily availability, chemical inertness and easy functionalization. Different types of cross-linker agents are incorporated into the PS resin matrix (PS-co-DVB) to improve the polymer rigidity, cross-linking density and surface area, such as DVB, EGDMA and TEGDA, the most common being DVB [10, 84].
For use of PS-co-DVB as catalytic support in different chemical reactions, it is desirable that it should have a small particle size, uniform size distribution, high porosity, cross-linked, high surface area, high activity and selectivity, high thermal, mechanical and chemical stability [85,86,87,88,89]. To achieve these properties, various synthesis parameters play a major role and by altering their amount and type, one can synthesize the desired polymer. Using organic polymer is highly advantageous as one can easily alter polymer properties during its synthesis, according to the desired application. From all the synthesis parameters available, some physicochemical parameters (stirring speed, temperature, monomer, initiator, cross-linker and diluent) are discussed briefly (Fig. 5).
Effect of agitation speed and temperature is well established in the literature. Particle size and agitation speed both are inversely proportional to each other. It is reported in the literature that agitation speed should have a range of 200–800 rpm; below and above this value, there will be rigorous conditions with rough particle surface and non-uniform distribution [90]. Further, higher the polymerization temperature smaller will be the polymer particle size. Also, there is a limit to the temperature range for polymerization (40-90 °C) [91,92,93,94]. Hence, agitation speed and temperature are major factors controlling the polymer particle size during their synthesis. Next, polymerization reaction time has no significant effect on polymer particle size and size distribution. However, type of monomer (hydrophilic/hydrophobic) affect the polymerization reaction mechanism. Selection of monomer depends upon the type of polymerization technique used, reaction medium and type of comonomer. During suspension polymerization, styrene and divinylbenzene (hydrophobic) and distilled water (reaction medium; polar in nature) are used, because hydrophobic monomers have high level of swelling and less retention for polar solvents. On the other hand, initiator, cross-linker and diluent have major effect on polymer particle properties, which are described in detail below.
Effect of initiator amount
Initiator is one of the elementary components used in the heterogeneous polymerization. Its nature and concentration decide the topochemistry of the initiation reaction [95]. The effects of the type of initiator on the kinetics of heterogeneous polymerization, on the stability of the reaction methods and on the size and size distribution of polymer particles are reported in the literature [52]. AIBN and BPO are the most widely used initiators in case of PS-co-DVB copolymer [48].
Kiatkamjornwong et al. [44] have reported the effect of initiator amount on polymer properties by changing the amount from 0.1 to 2.0 wt.% of the monomer concentration. The results shown that the conversion increased with initiator amount as more free radicals formed in the initiation period. At low initiator concentration, holes were generated on the particle surface because of crosslinking reaction; spherical particles with fusion were appeared at higher initiator concentration. Therefore, initiation rate affects the polymerization rate. Although, droplet coalescence can occur when the monomer droplets are not stabilized, which might show the insufficient amount of stabilizer in the monomer droplets.
Ober et al. [45] showed the effect of initiator amount at two different temperatures (68 °C and 75 °C) by changing the initiator amount from 1.0 to 2.0 g. In the first case, it was shown that the rate of free radical formation enhanced when the initiator amount increased, which leads to faster initial monomer consumption but also produced larger particle of lower molecular weight. At 75 °C, lower amount of initiator (0.55 g) was required to produce particles with smaller size, narrow size distribution and much higher molecular weight than at 68 °C.
The results revealed that at lower initiator amount (0.1 wt.%), particles produced with a rough surface and broader size distribution, however, at higher concentration (more than 2.0 wt.%), particles with smooth surface were obtained. It was shown that lower initiator amount could produce higher molecular weight polymer and vice versa. Also, the initiator amount was reduced when polymerization took place at higher temperature. Therefore, polymer particle size may increase or decrease on increasing the initiator amount and type of monomer used. Therefore, only a particular set of reaction conditions gave monodispersity with smaller size and with more or less initiator produced particles with wider distributions and the optimum particle uniformity may be obtained at some value in between lower and higher ones.
Effect of cross-linker amount
Cross-linking agents are used for the cross-linking among the monomer units, which affect the polymer properties. The effect of the type and amount of cross-linker agents on heterogeneous polymerization, particle surface morphology, size and size distribution and surface area, were examined by various researchers. Choice of type of the cross-linker agent depends upon the kind of monomer and polymerization technique employed. DVB, EGDMA and TEGDA are most widely used cross-linkers in case of PS-co-DVB copolymer [44].
Hulubei et al. [52] presented the effect of the cross-linker agent on polymer attributes by analyzing that porous structure starts to form after a critical value of cross-linker. The cross-linked polymers exhibited various morphologies depending on the diluent and cross-linker amount in the reaction mixture. As cross-linker amount increased in the reaction system, it leads to the creation of more rigid structures, with an increase of the pores and decrease of their size. At the same time, the cross-linker amount had a major effect on the surface area, which increases on increasing cross-linker content, and particle size, decreases with cross-linker content.
Mane et al. [96] reviewed the changes in polymer characteristics with cross-linking density. Higher cross-linking density improves the surface area and pore volume whereas decreases the pore size. Further, cross-linker increased thermostability, glass transition temperature and improved thermal properties. Low cross-linked polymers have the tendency of greater swelling whereas high surface area and pore volume encouraged the polymer swelling. Cross-linking agents offer insolubility, rigidity and stiffness to the polymer which proposed potential uses in various applications.
Therefore it was observed that higher the amount of cross-linker agent, greater the cross-linking density, higher the surface area, higher rigidity, improved thermal properties, decreased pore and particle size. Usually, during the experiments, the value of cross-linker agents varies from 0.1 to 40.0 wt.% of the monomer amount. At lower amount, polymer particles were produced polydispersed and fused because of insufficient crosslinking sites to maintain polymer particle sphericity. As content increased, particles produced were harder and tougher with smooth surface [97]. Thus, an optimum value will get after performing a number of experimental runs at various reaction conditions.
Effect of diluent amount and type
The polymer may be formed porous by the insertion of inert diluent (toluene, THF, n-hexane, n-heptane and MEK) in the monomer phase. The diluent is also called porogen i.e. a pore generating solvent, which attributes to polymer properties. The thermodynamic affinity of the diluent with the polymer is predicted by the solubility parameter and therefore, thermodynamic affinity finally controls the porous structure formation for polymer particles. The solubility parameter difference between monomer and diluent decides that which diluent should be considered a good or bad for a polymer. A diluent is considered a good solvent when this difference is zero or smaller than unity and a poor solvent when it is greater than unity. Both good and bad solvents have different extent of phase separation due to their different solvency which influences the polymer properties. Usually, it is said that higher the diluent solvating power, lower the porosity and higher the surface area [98,99,100,101,102].
Based on the literature survey, Toluene, THF and cyclohexane are considered good, among which, toluene gives the best result in case of PS-co-DVB copolymer (Table 2). Apart from the diluent type, the diluent amount is also one of the most significant factors which affect the polymer characteristics. The diluent amount with respect to monomer (monomer/diluent (v/v)) may vary from 1:1 to 1:6 or even more.
Mane et al. [103] redefined the effect of diluent on polymer properties and showed that the selection of a right porogen or a porogen pair (a pair of good and bad solvent) is an essential step before the synthesis of polymer particles because an improper porogen selection may give the low porosity polymer. The solvating porogen suggests a greater surface area (generation of micro/mesopores) although non-solvating porogen is able to generate a polymer with smaller surface area (generation of meso/macropores). Experiments were also performed to study the effect of the diluent amount by varying the monomer/diluent ratio from 1:1 to 1:4. After analysis of the results, it was revealed that particle size distribution was uniform in case of 1:1 and 1:2, while in other cases, non-uniform particle distribution was achieved. This type of performance may be due to the emulsion formation at the higher diluent amount, which did not allow polymer formation, also, there was coalescence, fusion and non-uniformity in size distribution.
Hao et al. [104] reported the effect of diluent content on polymer porosity and other properties. They have varied the diluent concentration from 0.66 to 7.0 (diluent/monomer (w/w)) and obtained the monodispersed polymer particles at 1.0 diluent/monomer content. With the increase of this value, the particle size distribution changed to polydispersity, especially above 3.0 diluent/monomer ratio.
Therefore literature results showed that good solvent has the smaller and greater miscibility in aqueous and organic phase, respectively, which allow the later phase separation between aqueous and organic phase, whereas bad solvent allows earlier phase separation. Thus, miscibility is not favored in case of bad diluent and it is separated out in an early stage. Also, the amount of diluent is very significant to synthesize the desired polymer, as optimal value achieved at around 1:1 monomer/diluent weight ratio, because at higher ratio emulsion formation were observed.
After studying the effect of various parameters during suspension polymerization, next section describes the control of particle size in brief. The major aim of the suspension polymerization technique is the formation of small size uniform dispersion of monomer droplets in the continuous phase with controlled coalescence of droplets. Droplets change from a low viscosity liquid to a sticky polymer-monomer mixture of increasing internal viscosity during the polymerization reaction and at last converted into porous polymer particles. Initially, the viscosity of monomer-polymer phase is low so breakage occurs easily, therefore breakage play an important role in controlling the droplet size at this time. But breakage becomes difficult as polymerization continues and viscosity increases. Now there is coalescence along with breakage which leads to an increment in droplet size because high viscosity limits the breakage of particles. After some time and above certain monomer conversion, there are no coalescence and polymer particles become hard [8]. Size distribution of initial monomer droplets and hence polymer particles depend upon the balance between break-up and coalescence of droplets, which is then controlled by the type and speed of the agitator, monomer and stabilizer used [105,106,107,108,109].
Advantages | Disadvantages |
|---|---|
• Easy removal of heat and control of temperature | • Wastewater problems |
• Polymer product with low impurity levels | • Lower productivity for the same reactor capacity |
• Low separation cost | • Polymer build up on the reactor wall |
• Low dispersion viscosity | • Difficulty in achieving higher conversion |
• Final product in particle form | • longer duration is required for higher conversion |
• Average molecular weight of 60,000 is possible | |
• Economical since water is used as heat transfer medium | |
• Isolation of product is easy due beads formation |
After concluding the revision of basic aspects of suspension method, effect of numerous synthesis parameters for PS-co-DVB copolymer particles, followed by advantages and disadvantages, next section of the article refer to the application of PS-co-DVB copolymer as catalyst support in various oxidation reactions in past 10 years.
Applications of PS supported catalysts in oxidation reactions
In industrial chemistry, catalytic oxidation reactions play an important role for the synthesis of numerous useful chemicals and intermediates (hydrocarbons, diols, alcohols and carbonyl). These reactions are carried out in the presence of various environment-friendly oxidants such as H2O2, TBHP and O2 [110,111,112]. Thus, great advances have been made in last few years for the development of new catalysts and efficient processes for several oxidation reactions.
Many catalytic supports such as silica, alumina, porous polymers, activated carbon and zeolites have been used for oxidation reactions [113, 114]. Organic polymers have been extensively explored as catalytic support during the last one decade as they are inert and can be easily functionalized. PS-co-DVB copolymer has shown outstanding activity in oxidation of various hydrocarbons and alcohols to aldehydes, ketones, epoxides and acids. Very first time in 1980, D. C. Sherrington has evolved that polymers can be used as catalysts as well as supports in chemical reactions [115]. In respect to polymeric supports, it was reported that about 65% of the reviewed catalytic systems are based on PS support and 15% are derived from PMMA and PEG and 20% are based on other systems [116,117,118,119]. The support that Merrifield used for his initial work (1984) in solid-phase peptide synthesis was based on 2% DVB cross-linked PS [120].
Various transition metals (Ru, Pt, Pd, Au and Ag) have shown high activity for liquid phase oxidation of alcohols [121,122,123]. For better catalytic activity, higher surface area, stability and dispersion of metal particles is required. Among various organic mesoporous materials, PS-co-DVB has shown high catalytic performance for alcohol oxidation [124]. Kaboudin et al. [125] reported the synthesis of AuNPs/PS-NH2 catalyst for selective oxidation of benzyl alcohol at mild reaction conditions (80 °C) by using air as an oxidant. Saadati et al. [74] investigated the performance of Cu (II) Schiff base complex supported on PS-co-DVB for catalytic oxidation of aldehydes using TBHP as oxidant. Recently, Wang et al. [126] reported the Fe-Co/S-PS supported catalysts for the oxidation of cyclic ketones using H2O2 as an oxidant. Table 3 summarizes the review of PS-co-DVB copolymer supported catalysts, synthesized by the suspension method, in the oxidation of alkenes, alkanes, alcohols, phenols, aldehydes and ethers.
Conclusions
-
A broad range of heterogeneous polymerization techniques such as suspension, emulsion, dispersion, precipitation and seeded polymerizations were discussed concisely. However, suspension polymerization technique is described in detail for understanding its fundamentals in a very simple way
-
Synthesis of polystyrene polymer was reviewed through suspension polymerization method and effect of various synthesis parameters was summarized during the copolymerization of PS-co-DVB
-
Porous PS-co-DVB copolymer has shown significant growth as catalyst support for various reactions due to their bulky pore diameter and uniform particle size distribution with a smooth surface
-
Lastly, PS-co-DVB copolymer supported catalysts have been discussed for various oxidation reactions and all the catalysts have shown high activity and selectivity for the desired product
Abbreviations
- PS:
-
Polystyrene
- DVB:
-
Divinyl Benzene
- AIBN:
-
Azobiz isobutyl nitrile
- THF:
-
Tetrahydrofuran
- PMMA:
-
Polymethyl methacrylate
- PEG:
-
Polyethylene glycol
- BPO:
-
Benzoyl peroxide
- EGDMA:
-
Ethylene glycol monomethyl ether
- TEGDA:
-
Tetraethyleneglycol diacrylate
- PVC:
-
Polyvinylchloride
- PVA:
-
Polyvinyl acetate
- PAAm:
-
Polyacrylamide
- TFE:
-
Tetrafluoro-ethylene
- ACN:
-
acrylonitrile
- PE:
-
Polyethylene
- PAc:
-
Polyacrylates
References
Gokmen MT, Prez FED (2012) Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog Polym Sci 37:365–405
Prasath RA, Gokmen MT, Espeel P, Prez FED (2010) Thiol-ene and thiol-yne chemistry in microfluidics: a straightforward method towards macroporous and nonporous functional polymer beads. Polym Chem 1:685
Qi S, Zhifeng D, Xiangju M, Shou XF (2015). Chem Soc Rev 44:6018
Dowding PJ, Goodwin JW, Vincent B (1998) The characterization of porous styrene–glycidyl methacrylate copolymer beads prepared by suspension polymerization. Colloids Surf A Physicochem Eng Asp 145:263–270
Heydarpoor S, Abbasi F, Jalili K, Najafpour M (2015) Synthesis of core-shell PS/PMMA expandable particles via seeded suspension polymerization. J Polym Res 22:151
El-Aassar MR, Soliman EA, Hashem AI, sun G, Amaly N (2017) Preparation and characterization of poly (styrene-co-Methacrylic acid) copolymer nanoparticles via precipitation polymerization. J Polym Res 24:207
Arshady R (1992) Suspension, emulsion, and dispersion polymerization: a methodological survey. Colloid Polym Sci 270:717–732
Tomovska R, Cal JC, Asua JM (2014). Wiley, New York, pp 59
Lima EV, Wood PE, Hamielec A (1997) An updated review on suspension polymerization. Ind Eng Chem Res 36:939–965
McNamara CA, Dixon MJ, Bradley M (2002) recoverable catalysts and reagents using recyclable polystyrene-based supports. Chem Rev 102:3275–3300
Hosseinzadeh S, Saadat Y, Abdolbaghi S, Taromi FA, Hosseinzadeh A (2014) Shape of the particles produced by seeded dispersion polymerization of styrene. Collod J 76:104–112
Kim JW, Suh K (2008) Monodisperse polymer particles synthesized by seeded polymerization techniques. J Ind Eng Chem 14:1–9
Kim JW, Suh K (2000) Monodisperse micron-sized polystyrene particles by seeded polymerization: effect of seed crosslinking on monomer swelling and particle morphology. Polymer 41:6181–6188
Lee K, Wi H (2010) Highly crosslinked micron-sized, monodispersed polystyrene particles by batch dispersion polymerization, Part 1: Batch, delayed addition, and seeded batch processes. J Appl Polym Sci 115:297–307
Qi Z, Han Y, Wang W, Song T, Chang J (2010). J Colloid Interface Sci 342:62
Kim JW, Ryu J, Suh K (2001) Monodisperse micron-sized macroporous poly(styrene- co -divinylbenzene) particles by seeded polymerization. Collod Polym Sci 279:146–152
Siddiqui MN, Redhwi HH, Vakalopoulou E, Tsagkalias I, Ioannidou MD, Achilias DS (2015) Synthesis, characterization and reaction kinetics of PMMA/silver nanocomposites prepared via in situ radical polymerization. Eur Polym J 72:256–269
Meouche W, Laatikainen K, Margaillan A, Silvonen T, Siren H, Sainio T, Beurroies I, Denoyel R, Branger C (2017) Effect of porogen solvent on the properties of nickel ion imprinted polymer materials prepared by inverse suspension polymerization. Eur Polym J 87:124–135
Ballard N, Aguirre M, Simula A, Leiza JR, Es S, Asua JM (2017) Nitroxide mediated suspension polymerization of methacrylic monomers. Chem Eng J 316:655–662
Asua JM (2018) Ostwald ripening of reactive costabilizers in miniemulsion polymerization. Eur Polym J 106:30–41
Schmidt BVKJ, Molinari V, Esposito D, Tauer K, Antonietti M (2017) Lignin-based polymeric surfactants for emulsion polymerization. Polymer 112:418–426
Sudjaipraparat N, Kaewsaneha C, Nuasaen S, Tangboriboonrat P (2017) One-pot synthesis of non-spherical hollow latex polymeric particles via seeded emulsion polymerization. Polymer 121:165–172
Silverstein MS (2017) Emulsion-templated polymers: Contemporary contemplations. Polymer 126:261–282
Cordoba CA, Collins SE, Passeggi Jr MCG, Vaillard SE, Gugliotta LM, Minari RJ (2018) Crosslinkable acrylic-melamine latex produced by miniemulsion polymerization. Prog Org Coat 118:82–90
Yamamoto T, Kawaguchi K, Takahashi Y (2016) Particle size control in the soap-free emulsion polymerization of styrene by an oil-soluble initiator with a weakly acidic water-soluble initiator. Colloids Surf A Physicochem Eng Asp 502:1–5
Oliveira PF, Machado RAF, Barth D, Acosta ED (2016) Dispersion polymerization of methyl methacrylate in supercritical carbon dioxide using vinyl terminated poly(dimethylsiloxane). Chem Eng Process 103:46–52
Chen R, Ren N, Jin X, Zhu X (2018) Stabilization capacity of PNIPAM microgels as particulate stabilizer in dispersion polymerization. Colloids Surf A Physicochem Eng Asp 538:789–794
Yang W, Hutchinson RA (2017) The influence of adding functionality to dispersant and particle core compositions in non-aqueous dispersion polymerization. React Funct Polym 114:31–37
Tan J, Li C, Dan S, Li H, Gu J, Zhang B, Zhang H, Zhang Q (2016). Chem Eng J 304:461
Wang X, Shen L, An Z (2018) Dispersion polymerization in environmentally benign solvents via reversible deactivation radical polymerization. Prog Polym Sci 83:1–27
Medeiros SF, Filizzola JOC, Oliveira PFM, Silva TM, Lara BR, Lopes MV, Bergmann BR, Elaissari A, Santos AM (2016) Fabrication of biocompatible and stimuli-responsive hybrid microgels with magnetic properties via aqueous precipitation polymerization. Mater Lett 175:296–299
Zhang H (2013) Controlled/“living” radical precipitation polymerization: a versatile polymerization technique for advanced functional polymers. Eur Polym J 49:579–600
Wang X, Huang P, Ma X, Du X, Lu X (2018) Magnetic mesoporous molecularly imprinted polymers based on surface precipitation polymerization for selective enrichment of triclosan and triclocarban. J Chromatogr A 1537:35–42
Huang H, Wang H, Wu Y, Shi Y, Deng J (2018) Chiral, crosslinked, and micron-sized spheres of substituted polyacetylene prepared by precipitation polymerization. Polymer 139:76–85
Jolly HC, Guillot P, Mignard E (2018) Supercritical continuous precipitation polymerization of acrylic acid in a droplet-based millifluidic device. Chem Eng J 334:389–399
Chaitidou S, Kotrotsiou O, Kotti K, Kammona O, Bukhari M, Kiparissides C (2008) Precipitation polymerization for the synthesis of nanostructured particles. Mater Sci Eng B 152:55–59
Kao YC, Whang WT, Chen YC, Chen KC (2017) Effect of crosslinking agents on the dispersive behaviour of polymer particles in seed swelling polymerisation. J Ind Eng Chem 51:216–222
Pei X, Zhai K, Liang X, Deng Y, Xu K, Tan Y, Yao X, Wang P (2018) Fabrication of shape-tunable macroparticles by seeded polymerization of styrene using non-cross-linked starch-based seed. J Colloid Interface Sci 512:600–608
Zhang Z, Shao H, Zhou X, Zhao L, Liu H, Ji X, Liu H (2017) Controllable synthesis of anisotropic silica/polymer composite particles via seeded dispersion polymerization. Mater Chem Phys 195:105–113
Yao Y, Cao Z, Shang Y, Chen Q, Yang L, Zhang Y, Qi D (2017) Preparation of polymeric/inorganic nanocomposite particles in miniemulsions: II. Narrowly size-distributed polymer/SiO2 nanocomposite particles. Colloids Surf A Physicochem Eng Asp 530:104–116
Kim D, Lee DY, Lee K, Choe S (2009) Effect of crosslinking agents on the morphology of polymer particles produced by one-step seeded polymerization. Macromol Res 17:250–258
Park J, Kim Y, Yoon H, Jun LBY (2011) Preparation of pore size controllable macroporous polymer beads. J Ind Eng Chem 17:794–798
Wang H, Xie G, Fang M, Ying Z, Tong Y, Zeng Y (2017) Mechanical reinforcement of graphene/poly(vinyl chloride) composites prepared by combining the in-situ suspension polymerization and melt-mixing methods. Composites Part B 113:278–284
Kiatkamjornwong S, Chientachakul P, Prasassarakich P, Damronglerd S (2001) Kinetic studies on styrene-divinylbenzene copolymerization by suspension technique. J Appl Polym Sci 82:1521–1540
Ober CK, Hair ML (1987). J Appl Polym Sci 25:1395
Kichatov BV, Korshunov AM, Assorova PV (2003). Theor Found Chem Eng 37:305
Liu Q, Wang L, Xiao A, Yu H (2010) The spherical cleavage behavior of Polydivinylbenzene during suspension polymerization. Des Mon Polym 13:369–375
Nunes DSS, Coutinho FMB (2002) Acrylonitrile–divinylbenzene copolymer beads: influence of pre-polymerization step, stirring conditions and polymerization initiator type on the polymer particle characteristics. Eur Polym J 38:1159–1165
Rodrigo R, Toro CA, Cuellar J (2013) Morphological characteristics of poly(styrene-co-divinylbenzene) microparticles synthesized by suspension polymerization. Powder Technol 247:279–288
Okudaira G, Kamogawa K, Sakai T, Sakai H, Abe M (2003). J Oleo Sci 53:167
Svec F, Frechet JMJ (1995) Temperature, a Simple and Efficient Tool for the Control of Pore Size Distribution in Macroporous Polymers. Macromol 28:7580–7582
Hulubei C, Vlad CD, Stoica I, Popovici D, Lisa G, Nica SL, Barzic AL (2014) New polyimide-based porous crosslinked beads by suspension polymerization: physical and chemical factors affecting their morphology. J Polym Res 21:514
Hoffman F, Delbruch K (1909) Patent (Ger.) No. 250 690, Farbenfabriken Bayer, Germany
Svec F, Frechet JMJ (1995). Biotechnol Bioeng 48:476
Viklund C, Svec F, Frechet JMJ, Irgum K (1996) Monolithic, “molded”, porous materials with high flow characteristics for separations, catalysis, or solid-phase chemistry: control of porous properties during polymerization. Chem Mater 8:744–750
Petro M, Svec F, Frechet JMJ (1996) Immobilization of trypsin onto “molded” macroporous poly(glycidyl methacrylate-co-ethylene dimethacrylate) rods and use of the conjugates as bioreactors and for affinity chromatography. Biotechnol Bioeng 49:355–363
Petro M, Svec F, Frechet JMJ (1996) Molded continuous poly(styrene-co-divinylbenzene) rod as a separation medium for the very fast separation of polymers comparison of the chromatographic properties of the monolithic rod with columns packed with porous and non-porous beads in high-performance liquid chromatography of polystyrenes. J Chromatogr A 752:59–66
Xie SF, Svec F, Frechet JMJ (1997) Preparation of porous hydrophilic monoliths: Effect of the polymerization conditions on the porous properties of poly (acrylamide-co-N,N?-methylenebisacrylamide) monolithic rods. J Polym Sci Part A Polym Chem 35:1013–1021
Peters EC, Svec F, Frechet JMJ (1997) Preparation of Large-Diameter “Molded” Porous Polymer Monoliths and the Control of Pore Structure Homogeneity. Chem Mater 9:1898–1902
Xie SF, Svec F, Frechet JMJ (1997) Rigid porous polyacrylamide-based monolithic columns containing butyl methacrylate as a separation medium for the rapid hydrophobic interaction chromatography of proteins. J Chromatogr A 775:65–72
Peters EC, Petro M, Svec F, Frechet JMJ (1998) Molded rigid polymer monoliths as separation media for capillary electrochromatography. 1. fine control of porous properties and surface chemistry. Anal Chem 70:2288–2295
Alexopoulos AH, Kiparissides C (2007) On the prediction of internal particle morphology in suspension polymerization of vinyl chloride. Part I. Chem Eng Sci 62:3970–3983
Tan L, Tan B (2016) Hypercrosslinked porous polymer materials: design, synthesis, and applications. Chem Soc Rev 46:3322–3356. https://doi.org/10.1039/c6cs00851h
Bauer W, Lauth H (1931) Patent (Ger.) No. 656, 134, Rohm and Hass, Darmstadt
Hosenstein WP, Mark H (1946) Polymerization of olefins and diolefins in suspension and emulsion. Part I. Int J Polym Sci 1:127–145
Trommsdorff E, Kohle H, Lagally P (1948) Zur polymerisation des methacrylsäuremethylesters1. Macromol Chem 1:169–198
Munzer M, Trommsdorff E (1997). High Polym 29:106
Yuan HG, Kalfas G, Ray WH (1991) Suspension polymerization. J Macromol Sci Rev Macrolol Chem Phys C 31:215–299
Okay O (2000) Macroporous copolymer networks. Prog Polym Sci 25:711–779
Kita R, Svec F, Frechet JMJ (2001) Hydrophilic polymer supports for solid-phase synthesis: preparation of Poly(ethylene glycol) methacrylate polymer beads using “classical” suspension polymerization in aqueous medium and their application in the solid-phase synthesis of hydantoins. J Comb Chem 3:564–571
Slater M, Snauko M, Svec F, Frechet JMJ (2006) “Click chemistry” in the preparation of porous polymer-based particulate stationary phases for μ-HPLC separation of peptides and proteins. Anal Chem 78:4969–4975
Mohammed ML, Mbeleck R, Saha B (2015) Efficient and selective molybdenum based heterogeneous catalyst for alkene epoxidation using batch and continuous reactors. Polym Chem 6:7308–7319
Mouradzadegun A, Mostafavi MA (2016) Copper-loaded hypercrosslinked polymer decorated with pendant amine groups: a green and retrievable catalytic system for quick [3 + 2] Huisgen cycloaddition in water. RSC Adv 6:42522–42531
Saadati F, Khani N, Rahmani M, Piri F (2016) Preparation and characterization of nanosized copper (II) oxide embedded in hyper-cross-linked polystyrene: highly efficient catalyst for aqueous-phase oxidation of aldehydes to carboxylic acids. Catal Commun 79:26–30
Taguchi Y, Suzuki T, Saito N, Yokoyama H, Tanaka M (2017) Preparation of polymer composite particles by phase separation followed by suspension polymerization. Open J Compos Mater 7:1–13
Alva G, Lin Y, Liu L, Fang G (2017) Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Buil 144:276–294
Dowding PJ, Vincent B (2000) Suspension polymerisation to form polymer beads. Colloids Surf A Physicochem Eng Asp 161:259–269
Liang YC, Svec F, Frechet JMJ (1997) Preparation and functionalization of reactive monodisperse macroporous poly(chloromethylstyrene-co-styrene-co-divinylbenzene) beads by a staged templated suspension polymerization. J Polym Sci Part A Polym Chem 35:2631–2643
Arshady R, Ledwith A (1983). React Polym 1:159
Minami H, Kojima A, Suzuki T (2017) Preparation of flattened cross-linked hollow particles by suspension polymerization in a solid dispersion medium. Langmuir 33:1541–1546
Erbay E, Bilgic T, Karali M, Savasci OT (1992) Polystyrene suspension polymerization: the effect of polymerization parameters on particle size and distribution. Polym-Plast Technol Eng 31:589–605
Lima EV, Hamielec AE, Wood PE (1994) Auto-acceleration effect in free radical polymerization. A comparison of the CCS and MH models. Polym React Eng 2:17–85
Villalobos MA, Hamielec AE, Wood PE (1993) Bulk and suspension polymerization of styrene in the presence of n-pentane. An evaluation of monofunctional and bifunctional initiation. J Appl Polym Sci 50:327–343
Grochowicz M, Gawdzik B (2013) Preparation and characterization of porous crosslinked microspheres of new aromatic methacrylates. J Porous Mater 20:339–349
Sharma S, Sinha S, Biswas P, Maurya MR, Chand S (2013) Oxidation of styrene over polymer- and nonpolymer-anchored Cu(II) and Mn(II) complex catalysts. J Appl Polym Sci 127:3424–3434
Sharma S, Sinha S, Chand S (2012) Polymer anchored catalysts for oxidation of styrene using TBHP and molecular oxygen. Ind Eng Chem Res 51:8806–8814
Sarkar S, Guibal E, Quignard F, SenGupta AK (2012) Polymer-supported metals and metal oxide nanoparticles: synthesis, characterization, and applications. J Nanopart Res 14:715
Dioos BML, Vankelecom IFJ, Jacobs PA (2006) Aspects of Immobilisation of catalysts on polymeric supports. Adv Synth Catal 348:1413–1446
Tank R, Gupta DC (2009) Modification of styrene-divinyl benzene copolymers using monoacrylates as ter-monomer. J Porous Mater 16:387–392
Apostolidou C, Stamatoudis M (1990) On particle size distribution in suspension polymerization of styrene. Collect Czechoslov Chem Commun 55:2244–2251
Portnikov D, Kalman H (2018) The effect of temperature on the mechanical characteristics of individual particles. Powder Technol 336:393–405
Azouz KB, Bekkour K, Dupuis D (2016) Influence of the temperature on the rheological properties of bentonite suspensions in aqueous polymer solutions. Appl Clay Sci 123:92–98
Ng WS, Cooper L, Connal LA, Forbes E, Jameson GJ, Franks GV (2018) Tuneable collector/depressant behaviour of xanthate-functional temperature-responsive polymers in the flotation of copper sulfide: effect of shear and temperature. Miner Eng 117:91–99
Zhenga T, Pilla S (2018) Encapsulation of hydrophilic payload by PU-PMF capsule: effect of melamine-formaldehyde pre-polymer content, pH and temperature on capsule morphology. Colloids Surf A Physicochem Eng Asp 542:59–67
Gritskova IA, Zhachenkov SV, Tsarkova MS, Levachev SM, Simakova GA, Khaddazh M, Prokopov NI (2011). Polymer Sci 53:568
Mane S, Ponrathnam S, Chavan N (2015). Can Chem Trans 3:473
Mane S, Ponrathnam S, Chavan N (2016). Can Chem Trans 4:192
Luz CTL, Coutinho FMB (2001). Polymer 42:4931
Daminova SS, Kadirova ZC, Sharipov KT, Stoyko OV, Chepulsky SA, Adewuyi A, Hojamberdiev M (2017) Diisopropyldithiophosphoric acid-impregnated macroporous non-ionogenic styrene-divinylbenzene polymeric sorbent (Porolas) for effective copper extraction. J Ind Eng Chem 55:204–214
Lu L, Jiang C, Xiufang W, Pihui P, Zhuoru Y (2006). Chin J Chem Eng 14:471
Kiatkamjornwong S, Traissaranapong S, Prasassarakich P (1999). J Porous Mater 6:215–229
Aungsurpravate O, Kangwansupamonkon W, Chavasiri W, Kiatkamjornwong S (2007). Polym Eng Sci 447
Mane S (2016). Can Chem Trans 4:210
Hao D, Gong F, Wei W, Hu G, Ma G, Su Z (2008) Porogen effects in synthesis of uniform micrometer-sized poly(divinylbenzene) microspheres with high surface areas. J Colloid Interface Sci 323:52–59
Calabrase RV, Chang TPK, Dang PT (1986) Drop breakup in turbulent stirred-tank contactors. Part I: Effect of dispersed-phase viscosity. AICHE J 32:657–666
Borwankar RP, Chung SI, Wasan DT (1986). J Appl Polym Sci 329:5749
Bourne JR, Baldyga (1994). J Chem Eng Sci 499:1077
Coulaloglou CA, Tavlarides LL (1976). AICHE J 229:289
Doulah MS (1975). Ind Eng Chem Fundam 149:137
Chaudhary V, Sweta (2016). IJSER 7:1743–1748
Chaudhary V, Sweta (2017). J Porous Mater 24:741–749
Fu Y, Xu L, Shen H, Yang H, Zhang F, Zhu W, Fan M (2016) Tunable catalytic properties of multi-metal–organic frameworks for aerobic styrene oxidation. Chem Eng J 299:135–141
Tamami B, Ghasemi S (2011) Modified crosslinked polyacrylamide anchored Schiff base–cobalt complex: a novel nano-sized heterogeneous catalyst for selective oxidation of olefins and alkyl halides with hydrogen peroxide in aqueous media. Appl Catal A Gen 393:242–250
Godhani DR, Nakum HD, Parmar DK, Mehta JP, Desai NC (2016) Tuning of the reaction parameters to optimize allylic oxidation of cyclohexene catalyzed by zeolite-Y entrapped transition metal complexes. J Mol Catal A Chem 415:37–55
Sherrington DC (1980). British Polym J 70
Kralik M, Corain B, Zecca M (2000). Chem Pap 54:254
Arnold U (2008) Mechanisms in homogeneous and heterogeneous epoxidation catalysis, p 387
Collma JP, Kosydar KM, Bressan M, Lamanna W, Garrett T (1984) Polymer-bound substrates: a method to distinguish between homogeneous and heterogeneous catalysis. J Am Chem Soc 106:2569–2579
Gravert DJ, Janda KD (1997) Organic synthesis on soluble polymer supports: liquid-phase methodologies. Chem Rev 97:489–510
Merrifield B (1984) The role of the support in solid phase peptide synthesis. British Polym J 16:173–178
Yamaguchi K, Mizuno N (2003) Scope, kinetics, and mechanistic aspects of aerobic oxidations catalyzed by ruthenium supported on alumina. Chem Eur J 9:4353–4361
Abad A, Concepcion P, Corma A, Garcia H (2005) A collaborative effect between gold and a support induces the selective oxidation of alcohols. Angew Chem Int Ed 44:4066–4069
Jamwal N, Gupta M, Paul S (2008) Hydroxyapatite-supported palladium (0) as a highly efficient catalyst for the Suzuki coupling and aerobic oxidation of benzyl alcohols in water. Green Chem 10:999
Renuka MK, Gayathri V (2018) A polymer supported Cu(II) catalyst for oxidative amidation of benzyl alcohol and substituted amines in TBHP/H 2 O. Catal Commun 104:71–77
Kaboudin B, Khanmohammadi H, Kazemi F (2017) Polymer supported gold nanoparticles: synthesis and characterization of functionalized polystyrene-supported gold nanoparticles and their application in catalytic oxidation of alcohols in water. Appl Surf Sci 425:400–406
Wang Y, Huang J, Xia X, Peng X (2018) Fe–Co/sulfonated polystyrene as an efficient and selective catalyst in heterogeneous Baeyer–Villiger oxidation reaction of cyclic ketones. J Saudi Chem Soc 22:129–135
Gupta KC, Sutar AK (2008) Polymer supported catalysts for oxidation of phenol and cyclohexene using hydrogen peroxide as oxidant. J Mol Catal A Chem 280:173–185
Hatefi M, Moghadam M, Sheikhshoaei I, Mirkhani V, Tangestaninejad S, Baltork IM, Kargar H (2009) Ru(salophen)Cl supported on polystyrene-bound imidazole: an efficient and robust heterogeneous catalyst for epoxidation of alkenes with sodium periodate. Appl Catal A 370:66–71
Krishnan GR, Sreekumar K (2009) Polystyrene-supported poly(amidoamine) dendrimer–manganese complex: synthesis, characterization and catalysis. Appl Catal A 353:80–86
Chang Y, Lv Y, Lu F, Zha F, Lei Z (2010) Efficient allylic oxidation of cyclohexene with oxygen catalyzed by chloromethylated polystyrene supported tridentate Schiff-base complexes. J Mol Catal A Chem 320:56–61
Tangestaninejad S, Moghadam M, Mirkhani V, Baltork IM, Torki M (2011) Preparation and characterization of molybdenum hexacarbonyl encapsulated in polystyrene and its application as an efficient and reusable catalyst for epoxidation of alkenes with tert-BuOOH. C R Chimie 14:604–610
Mohammed ML, Mbeleck R, Patel D, Niyogi D, Sherrington DC, Saha B (2015) Greener and efficient epoxidation of 4-vinyl-1-cyclohexene with polystyrene 2-(aminomethyl)pyridine supported Mo(VI) catalyst in batch and continuous reactors. Chem Eng Res Des 94:194–203
Zhu Y, Zhao W, Hosmane NS (2015) Direct synthesis of carboranylpolystyrene and their applications for oxidation resistance of graphene oxides and catalyst support. J Organomet Chem 798:80–85
Sharma AS, Kaur H (2017) Au NPs@ polystyrene resin for mild and selective aerobic oxidation of 1,4 dioxane to 1,4 dioxan-2-ol. Catal Commun 90:56–59
Khatun R, Biswas S, Ghosh S, Islam SM (2018) Polymer-anchored [Fe(III)Azo] complex: an efficient reusable catalyst for oxidative bromination and multi-components reaction for the synthesis of spiropiperidine derivatives. J Organomet Chem 858:37–46
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaudhary, V., Sharma, S. Suspension polymerization technique: parameters affecting polymer properties and application in oxidation reactions. J Polym Res 26, 102 (2019). https://doi.org/10.1007/s10965-019-1767-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-019-1767-8