Abstract
Silane coupling agents are potential reagents widely used to improve the compatibility between silica and less polar rubber, especially natural rubber (NR). Nevertheless, high temperature is generally required to generate the interaction between the components during the mixing process. Accordingly, an alternative method by grafting the silane coupling agent onto the rubber molecules would be a desirable approach to develop a compatibilizer for the silica-filled NR compound. In this work, skim NR was used as a starting material due to its linear structure. The optimal conditions of the grafting reaction were found to be 1 phr of an alkoxy silane and 5 phr of benzoyl peroxide under 8 min of UVA irradiation time. These conditions were applied for producing the rubber material used in the mixing process of STR 5L and silica. The cure characteristics, silica dispersion and mechanical properties of the rubber compounds were improved, suggesting that the modified rubber was an efficient material for increasing the compatibility between silica and NR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the rubber technology, carbon black and silica have been used as reinforcing fillers to improve the performance of the rubber products. Carbon black is the common filler carried out in the rubber mixing process due to the facile operation and the good mechanical properties of the obtained products [1]. Silica is an alternative reinforcing filler which gains more potential compared to carbon black in the reinforcement of elastomers, especially in the tire industry. It can improve the tear strength, abrasion resistance and rolling resistance of the rubber tires [2, 3]. However, the silica-filled natural rubber (NR) compounds usually have a problem about the incompatibility due to the different polarity between NR and silica, affecting the mechanical properties of the rubber products. There are various techniques used to enhance the miscibility of silica in NR such as the addition of silane coupling agent as well as chemical modification of the silica surface and NR structure [4,5,6].
TESPT (bis(3-triethoxysilylpropyl)-tetrasulfide) is a well-known silane coupling agent widely used in the rubber processing [7]. It is a sulfur-containing organosilane which can enhance the cure characteristics of the NR compound due to its ability to promote sulfur atoms for crosslinking during the vulcanization process [8]. The properties and processability of the compounds were also improved by the incorporation of the TESPT in the suitable curing system [9]. However, the restriction was found to be the requirement of the high-temperature reaction for TESPT and rubber, causing the degradation of the rubber [10]. For the sol-gel method, the dispersion of filler increased because this method directly produced the silica particle in the rubber matrix. Besides, the silica surface can be modified in order to obtain the desired properties, i.e., introducing the silane coupling agent onto its surface to solve the cure retardation [11, 12]. Nevertheless, the complicated reaction is required for this technique, leading to an inappropriate for the practical usage. Introducing the polar molecules onto the NR molecules is another good method to increase the miscibility of silica and NR. However, it was found that the cure behaviors of the compound were retarded by the presence of the new molecule on NR [13]. Thus, the efficient technique which can improve filler-rubber interaction as well as cure characteristics should be investigated. The advantages of the silane coupling agent and NR modification were applied to develop the alternative method. The grafting reaction of the silane coupling agent onto the NR molecule considered to be a reasonable approach.
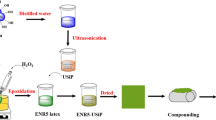
In this work, skim NR (SNR), which is a by-product from a manufacturing process of the concentrated NR latex, would be applied as a starting material in a grafting reaction in order to increase its benefit and improve its properties for use in the mixing process. In the rubber industry, SNR is usually considered waste with poor mechanical properties because of its high amounts of non-rubber components [14]. Nevertheless, its linear molecular structure is attractive for potential chemical modifications [15]. By comparing to the branched molecule of the regular NR, the linear molecule of SNR should react more easily for steric reasons. An alkoxy silane containing the C=C, KBM-502, was used for the first time for NR. KBM-502 was grafted onto the SNR molecules because it contains the appropriate site to react with the SNR molecules. The chemical structure of KBM-502 is illustrated in Fig. 1. The reaction was initiated by the induction of UVA irradiation, which is a convenient modification method [16, 17]. The reaction is not only easy to handle, but also fast and clean. The grafting reaction was performed in the latex state due to many advantages, including low toxic condition [18]. The grafted rubber would then be used as a material, which increasing the compatibility between STR 5L and silica in the mixing process, as proposed in Fig. 2.
Experiment
Preparation of grafted saponified skim NR (G-SSNR)
Fresh SNR latex (Thai Rubber Latex Co., Ltd.) (about 4% dry rubber content (DRC)) was preliminarily recovered by adding a 0.37% w/w solution of sodium alginate (Carlo erba reagents) and stirred for 1 h, then left to stand for 24 h. The creamed SNR latex (about 18% DRC), which appeared in the upper fraction, was subjected to saponification by adding a 1% w/w solution of SDS (VWR International Co., Ltd.) and a 1% w/w solution of NaOH (Labscan Asia Co., Ltd.) and the mixture was stirred at 70°C for 3 h. These steps were carried out in order to eliminate the proteins present in the latex, which can serve as a free-radical scavenger in the reaction [19, 20]. The saponified skim NR (SSNR) latex was neutralized using a 10% v/v solution of HCl (VWR International Co., Ltd.) and then diluted with distilled water to achieve the DRC of 10%. The saponified SNR (SSNR) latex was then subjected to a grafting reaction.
Benzoyl peroxide (BPO) (Merck Co., Ltd.) and KBM-502 (3-methacryloxypropylmethyldimethoxysilane) (Shin-Etsu Chemical Co., Ltd.) were used as a photo-initiator and silane coupling agent, respectively, in the form of emulsions. As the medium of SSNR latex was water, the organic-based chemicals could not dissolve in the system. Thus, the emulsions of KBM-502 and BPO in distilled water were required in the hopes of increasing their compatibility with the SSNR. A BPO mixture was prepared by adding distilled water (7 mL) to a solution of BPO (0.25 g) in toluene (3 mL). KBM-502 (250 μL) was mixed with distilled water (10 mL). Both mixtures were freshly emulsified using a mechanical homogenizer (UH-3C, Sonics) separately. Emulsions of KBM-502 and BPO were used as stock reagents to study the effect of their amounts by adding to the SSNR latex (10 g) and stirred for 1.5 h at room temperature to increase the homogeneity. The variable parameters affecting grafting efficiency of silane coupling agent onto SSNR molecule were studied, as presented in Table 1. Each rubber mixture was then transferred to a petri dish and exposed to the UVA irradiation to obtain the G-SSNR latex. The UVA lamp used in this experiment was high pressure mercury UV lamp (RUV 2525 BP). The G-SSNR latex was then coagulated with acetone (RCI Labscan Ltd.) and dried at 50°C. Each sample was further purified by reprecipitation in toluene/methanol (RCI Labscan Ltd.) and dried at 50°C until its weight was unchanged. This step was performed to ensure that the new signals appeared in the FTIR and NMR analyses were solely from the new compounds, not the mixture of KBM-502 and BPO.
Rubber compounding
Three conditions of rubber compounds were investigated, as shown in Table 2. In condition 2, KBM-502 was directly added at the same amount as KBM-502 grafted onto the SSNR molecules in the STR 5L (Standard Thai Rubber grade 5L) (Pan Star Co., Ltd.) blended with G-SSNR system. It was observed for clarifying the influence of the chemical bonding in the G-SSNR molecules. For the mixing process, a two-stage mixing procedure was carried out in order to prevent premature vulcanization. An internal mixer (HAAKE™, Rheocord 9000 Fisons) and a two-roll mill (W 100 T, DR. Collin GMBH) were employed for preparing the rubber compounds. The mixing conditions were set as follows: fill factor = 0.8, initial chamber temperature = 40°C and rotor speed = 40 rpm. Firstly, the rubbers were charged into the mixer and masticated for 2 min. Silica powder (HiSil® 233) (OSC Silica Co., Ltd.) was added portionwise while mixing for the total mixing time of 11 min, followed by an addition of ZnO (zinc oxide) (Thai-Lysaght Co., Ltd.) and stearic acid (Acidchem International SDN). The mixing was continued for 2 min to finish the first step. The resulting rubber compounds were taken out of the mixer and sheeted using the two-roll mill to cool down the compounds and the chamber of the internal mixer. After adding the vulcanizing agents (sulfur and TBBS) (The Siam Chemical Public Co., Ltd.), the mixture was further mixed for 2 min in the internal mixer. TBBS (n-tert-butyl-2-benzothiazyl sulfonamide) was incorporated with sulfur as an accelerator. The rubber compounds were finally discharged and then sheeted using the two-roll mill. In condition 2, KBM-502 was added and mixed for 2 min before the addition of ZnO and stearic acid.
Characterization and properties measurement
The chemical structure of the purified G-SSNR was analyzed by FTIR in attenuated total reflection (ATR) mode (JASCO: FT-IR 4100). The dried film of each sample was placed on a germanium (Ge) disk and analyzed with 100 scans at a resolution of 4 cm−1.
The structure of G-SSNR was confirmed by 1H–NMR (Bruker 500 UltraShield™) and 29Si-NMR (Bruker Ascend™ 400) experiments. Approximately 30 mg of each rubber sample was dissolved in toluene-d 8 for both NMR analyses.
The content of KBM-502 on the grafted rubber molecules was determined by FTIR using a calibration curve (Eq. (1)) constructed using KBM-502 (0, 3, 6 and 9 phr) in purified synthetic cis-1,4-polyisoprene as a standard. The dried films of mixtures were then placed on the Ge disk and analyzed using FTIR spectrometer in the ATR mode. The intensity ratios of the carbonyl (C=O) absorption peak of KBM-502 at 1721 cm−1 (A1721) to that of the methyl (CH3) of NR at 1375 cm−1 (A1375) against 4 known concentration of KBM-502 was obtained. In order to observe the amount of the grafted KBM-502 on the G-SSNR molecules, the intensity ratio of each purified G-SSNR was recorded by FTIR and then converted to KBM-502 content using the following equation:
where Y is A1721/A1375 and X is the quantity of KBM-502 content (phr).
The percentage of grafting efficiency was calculated in order to compare the capability of KBM-502 grafting onto the rubber molecules in various loading of KBM-502 using Eq. (2).
Gel content of each rubber sample (0.03 g) was determined by dissolving in toluene (30 mL) and kept in the dark at room temperature for 7 days. After that, the insoluble or gel fraction was collected and coagulated using methanol. The gel was dried at 70°C until its weight became constant. The percentage of the gel content was calculated using the following equation:
where W1 is the weight of initial dry rubber sample and W2 is the weight of dried gel.
Rubber processing analyzer (Alpha Technologies RPA 2000) was employed to determine the degree of filler-filler interaction (Payne effect) using strain sweep test. The strain sweep test (0.5–1000%) was carried out by varying the degree of angular oscillation from 0.02 to 90° of arc at the test chamber temperature of 100°C and a constant frequency of 1 rad/s.
Bound rubber content was determined to observe the rubber-filler interaction. Approximately 0.2 g of each uncured rubber compound was cut into small pieces. The specimen was placed in toluene (25 mL) at room temperature for 14 days. The solvent was changed by removing the original (20 mL) and replacing with the new solvent (toluene, 20 mL) after 7 days in order to dissolve all unbound rubbers in the sample. Then, the bound rubber was collected on a filter paper and dried at 70°C until the weight was unchanged. The bound rubber was calculated using the following equation:
where Wfg is the weight of silica and gel. W is the weight of rubber compound and mf is the weight of filler in the compound.
Moving die rheometer (Tech pro rheotech MD+) was employed to evaluate the cure characteristics of the rubber compound. Approximate 5 g of each uncured rubber compound was placed on a rheometer cavity disk at 150°C under the oscillating arc of 0.5 or 1 degree.
The tensile properties of the vulcanized rubber compounds were determined using a universal extensometer (Instron Model 5566) with a cross head speed of 500 mm/min and load cell of 1 kN. The vulcanized rubber sheets with an approximate thickness of 1 mm were stamped out using a type C dumbbell die to obtain test pieces in accordance with ASTM D 412.
Crosslink density was investigated by the swelling test. Approximately 0.7 g of vulcanized rubber compounds were cut into small pieces. The specimen was immersed in toluene (100 mL) for 7 days at room temperature in the dark. Then, the swollen samples were collected and excess liquid on the specimen surface removed using filter paper. The specimens were weighed until the weight became stable. Crosslink density (ν) was calculated using the following equations:
where ν is crosslink density and Mc is molecular weight between crosslink. χ is Huggins interaction constant (0.38 for silica-filled NR).V0 is the molar volume of the solvent (106.9 cm3/mol for toluene). The value of ρr and ρs are 0.93 g/m3 and 0.886 g/m3, respectively. θ is volume fraction. ws and wu are the weight of the specimen and weight of the swollen rubber, respectively. c is a parameter for silica-rubber interaction.
Fracture surface morphology of each vulcanized rubber compound was observed using a scanning electron microscopy, SEM (JEOL JSM5410LV). The fracture surface was initiated by a cryogenic fracturing process in liquid nitrogen for 5–10 min and then coated with palladium (Pd). The operation was at accelerating voltage of 20 kV under the high vacuum (HV) mode. The working distance was 20 mm.
Results and discussion
Structural characterization of G-SSNR
The FTIR spectra of the SSNR and the G-SSNR are shown in Fig. 3(a) and (b), respectively. The important characteristic bands of the SSNR appeared at 1664 and 1375 cm−1, which corresponded to the C=C and methyl (–CH3) stretchings in the NR molecule, respectively [21]. If the KBM-502 was successfully grafted, a new carbonyl (C=O) stretching should be observed at 1721 cm−1. Pleasingly, it was found that the new absorption band at 1721 cm−1 was observed in the purified G-SSNR.
The structure of the purified G-SSNR was further analyzed by the 1H–NMR technique (Fig. 4). The characteristic chemical shifts (δ) of the cis-1,4-polyisoprene unit appeared at (c) 1.75, (d) 2.09 and (b) 5.25 ppm, corresponding to the methyl, methylene and unsaturated methine protons, respectively [22]. The KBM-502 unit grafted onto the SSNR backbone was suggested by the signal at (a) 3.55 ppm, which is characteristic of the methoxy protons from KBM-502. This result also supported the accomplishment in the grafting of KBM-502 onto the SSNR backbone. However, other signals from KBM-502 could not be clearly seen because they were obscured by the signals from the SSNR structure itself and toluene-d 8. Moreover, the amount of KBM-502 grafted onto the G-SSNR was very small compared to the structure of G-SSNR as whole, making it much more difficult to observe.
The presence of the silicon atoms in the G-SSNR molecules was confirmed by 29Si-NMR, as shown in Fig. 5. After purification, only one additional singlet was observed at 4.93 ppm, corresponding to the silicon atom from the KBM-502 unit. Tetramethylsilane (TMS) was added as a reference. The chemical shift of G-SSNR was higher than that of TMS because the silicon atom in the G-SSNR (b) was much deshielded by the methoxy groups. This result confirmed the possession of silicon atoms on the G-SSNR purified in methanol. This suggested that KBM-502 was successfully attached on the SSNR backbone by the grafting reaction.
A mechanism of grafting radical-induced diene molecules onto a polyolefin backbone has been proposed to indicate that the grafting reagent could add in place of either allylic protons [21, 23]. Moreover, the π bond of C=C could undergo dissociation to abstract a radical species in the system [24]. In this work, BPO as a photo-initiator, decomposes homolytically under UVA irradiation and then into phenyl radicals. The phenyl radicals generated could then initiate the reaction, which may occur in two different ways. One is the abstraction of either of the allylic protons ((a) and (b)) and the other is the addition of the radical species onto C=C of the SSNR backbone ((c)) (Fig. 6). Each allylic proton on the SSNR was prone to being abstracted by the radical initiator, resulting in two possible positions being grafted ((a) and (b)). Also, the addition of the radical initiator onto the C=C of the SSNR backbone could give rise to the addition of the KBM-502 unit onto the SSNR structure ((c)).
Parameters affecting the grafting efficiency of the silane coupling agent
The various amounts of KBM-502 from 1 to 9 phr in the presence of 3 phr of BPO for 8 min of UVA irradiation were optimized to study the effect of the concentration of the silane coupling agent. This condition was applied from the modification of NR reported in the literature [24]. It was found that the increase in the KBM-502 loading did not significantly affect the KBM-502 grafted content, as shown in Fig. 7. Since the proportion of the KBM-502 grafted was quite low (0.5–0.7 phr), this might limit the number of active sites on the rubber backbone generated by the initiator (BPO). This reason was consistent with the mechanism of the reaction proposed, as shown in Fig. 6 where the reaction initially occurred by induction of the radical-initiator, followed by the radical chain reaction to react with KBM-502. Hence, the grafting efficiency was not affected by the concentration of the silane coupling agent. Since the KBM-502 content grafted was not remarkably different in each sample, it was decided to use the grafting efficiency (%) to determine the optimal concentration of KBM-502 for use in the grafting reaction. The highest value was observed at 1 phr of the KBM-502 loading. Therefore, the KBM-502 concentration of 1 phr was the optimum and would be used when studying the effects of other parameters on the grafting reaction.
The effect of the photo-initiator concentration was determined in the presence of BPO of varying amounts between 1 and 9 phr under UVA irradiation of 8 min. The KBM-502 loading used in this experiment was 1 phr which was the optimal concentration previously examined. In Fig. 8 (striped bar), it could be seen that the minimum amount of BPO required to start the reaction was 3 phr. This suggested that BPO was the factor controlling the proliferation of the grafting reaction. The amount of the KBM-502 grafted onto the rubber backbone increased with increasing BPO concentration and remained unchanged after 5 phr. This could be explained by the fact that the increase in the amount of the initiator could generate more active sites on the rubber backbone for grafting KBM-502 onto. However, the grafting efficiency was not affected by the addition of BPO higher than 5 phr. The excess amount of BPO did not increase the interaction between the rubber and KBM-502. Furthermore, the competing reaction could possibly occur by the active sites generated by the initiator where the interaction between the rubber molecules resulted in the formation of a branched network. It was confirmed by the gel contents as given in Fig. 8 (black bar). The gel content increased with increasing BPO concentration and stayed steady from 5 to 9 phr of BPO. This suggested that branching was limited by the steric effect, so the gel formation did not increase when the BPO concentration was higher than 5 phr. Thus, the optimal concentration of BPO was at 5 phr.
The effect of the UVA irradiation time was studied over a range of 2–12 min. The reaction was carried out using 1 phr of KBM-502 and 5 phr of BPO. The KBM-502 content grafted onto the SSNR molecules at various reaction times is shown in Fig. 9 (striped bar). It was found that the grafting reaction occurred after 4 min. The amount of the KBM-502 incorporated into the rubber molecules increased with increasing duration of UVA exposure to attain the maximum value at 8 min. This implies that 8 min of UVA irradiation was sufficient for the grafting reaction. However, it was found that the grafting efficiency decreased thereafter. This suggested that the longer the irradiation time was, the more possibility of collision between the radical species to take place would be. Nevertheless, the crosslinking which occurred from the competing side reaction between the rubber molecules was observed as the increase in the gel content (8–12 min of UVA irradiation time), as shown in Fig. 9 (black bar). This may be because the radicals in the allylic position of the SSNR backbone after exposure to UVA light could react with unsaturated carbon of other SSNR molecules, as proposed in Fig. 10.
Thus, the reaction in the presence of 1 phr of KBM-502 and 5 phr of BPO under 8 min of UVA irradiation time was the optimal condition to produce G-SSNR.
Rubber compounding
G-SSNR produced from the optimal grafting reaction was subjected to use as a material to increase the compatibility in a mixing process. The presence of chemical bonding between the silane coupling molecules and SSNR from the grafting reaction was supposed to be an advantage in the mixing system.
The cure behaviors, i.e., scorch time and cure time, of the rubber compounds were analyzed using the cure curve from built-in software, as presented in Table 3. The results showed that the incorporation of G-SSNR substantially decreased the cure time (Entry 3). On the other hand, it was found that the compound with KBM-502 added as a sole compatibilizer exhibited a high cure time which showed no difference from that without a compatibilizer. It might be due to the existence of the chemical bonding in the G-SSNR to increase the cure rate. As the adsorption of the curatives was a major issue in the silica-filled rubber compound, the presence of the chemical bonding in G-SSNR could be a key to reduce this disadvantage [25]. The proposed interaction of each component in the compounds is illustrated in Fig. 11. In the KBM-502 added STR 5L system, the silane coupling agent was directly added into the rubber compound during the mixing process. Since the polarity of each ingredient was different, KBM-502 would prefer to combine with themselves. This could be explained by the like-dissolves-like rule. Therefore, the free KBM-502 formed droplets in the rubber matrix instead of trapping the surface of silica. The curing agent in the STR 5L with KBM-502 system was still adsorbed by silica due to the similarly high polarity, resulting in no improvement of the cure rate in this process. In contrast, the fusion of the silane coupling molecules in the case of G-SSNR could not occur because they were fixed on the SSNR backbones by chemical bonding, leading to greater efficiency to interact with silica particles. The silane coupling molecules attached on the SSNR could be well dispersed in the rubber matrix in the masticating step. This is due to the good compatibility between STR 5L and the rubber part of the G-SSNR. After adding silica, the coupling agent dispersed in the rubber matrix could readily react with the silica particles, leading to a decrement of the silanol groups. A better cure rate was observed as the accelerators adsorbed by silica decreased. Additionally, the low vulcanization rate occurred not only because of the adsorption of the curing agent, but also of more time required to form the network in the compounds during vulcanization. In the case of STR 5L mixed with G-SSNR, linkages between the rubber and the silane coupling molecules were present, so the period to form the networks in vulcanization was shortened. Furthermore, the presence of both free KBM-502 and G-SSNR decreased the scorch time of the rubber compound. This suggested that the incorporation of the silane coupling agent into the silica-filled rubber compound could accelerate the vulcanization in the earliest stage. However, KBM-502 could not be well function in the curing stage, observed from the high cure time of STR 5L added with KBM-502 system (Entry 2). The cure curves of the rubber samples are provided in the supporting information.
From Fig. 12, the results showed that STR 5L exhibited the lowest degree of the crosslink density. After adding KBM-502, the crosslink density did not significantly increase. However, the incorporation of G-SSNR raised the degree of crosslinking of the rubber compounds. This phenomenon corresponded to the cure characteristic results where the KBM-502 grafted onto SSNR enhanced the cure state of the rubber compounds while the free KBM-502 could not improve this behavior. Additionally, better interaction in the rubber compound also involved to the resistance in swelling, indicating the enhancement in crosslink density [26, 27].
The filler-filler interaction can be indicated by measuring the elastic modulus (G’) at low strain, known as the Payne effect [28, 29]. From Fig. 13, the compound without a compatibilizer (STR 5L) exhibited the highest elastic modulus at low strain which indicated a high degree of silica aggregation. In the system of STR 5L and KBM-502, it was found that the elastic modulus was not significantly different from that of STR 5L. On the contrary, the modulus decreased markedly in the case of STR 5L mixed with G-SSNR, indicating a low degree of the Payne effect. From this piece of evidence, it was found that the presence of chemical bonding in G-SSNR was a major factor in reducing the filler-filler interaction. The droplets of KBM-502 were formed, decreasing the ability to react with the silanol groups on the surface of silica. However, the presence of the covalent bonding between KBM-502 and SSNR in G-SSNR solved this problem as the silane coupling agent attached on the rubber matrix could interact with the silica better. The silanol groups on the silica surface were trapped by the hydrolyzable part of the silane coupling molecules. This was related to the explanation of the cure characteristics. By trapping the silanol, silica did not only reduce the adsorption of the curing agents, but did also decrease in the aggregation.
Bound rubber is another important parameter in the reinforcement aspect which is normally occur through both chemical and physical interactions between rubber and reinforcing fillers [4, 30]. From Fig. 14, it can be seen that STR 5L exhibited the lowest content of bound rubber, indicating the lowest filler-rubber interaction. This could be because of the difference in the polarity between NR and silica, resulting in a decrease in the compatibility between these two components. For STR 5L added with KBM-502, the bound rubber content was not remarkably different from that of STR 5L. However, the STR 5L mixed with G-SSNR sample exhibited the increment of the bound rubber content, suggesting the escalation of the filler-rubber interaction. These findings are consistent with the results of the filler-filler interaction. The chemical interaction in the G-SSNR could decrease the interaction between silica particles as described above. The decrease in filler-filler interaction led to the enhancement of the filler-rubber interaction. The presence of the covalent bond in the G-SSNR implies the linkage between NR and silica, leading to the better NR-silica compatibility.
The cryogenic fracture surface of the vulcanized rubber compounds was prepared in order to determine the dispersion of silica in the rubber matrix. SEM micrograph of each sample is illustrated in Fig. 15. The gray sphere and black background indicate the silica particles and the rubber matrix, respectively. From the results, the formation of silica agglomerates was observed in the condition without a compatibilizer (STR 5L) (a). This observation was consistent with the result from the Payne effect which exhibited high modulus at low strain, indicating a high degree of filler-filler interaction. This was because the high polarity of silica surface could cause the aggregation of the filler. The morphology of STR 5L mixed with KBM-502 is shown in micrograph (b). The silica agglomeration also appeared in this system, which could be explained in the same manner as that in the STR 5L system. Nevertheless, the well-dispersion of silica in the rubber matrix was observed when G-SSNR was incorporated, as shown in micrograph (c). The large agglomerates of silica were not found in this rubber matrix.
Moduli at 100 and 300% strain of rubber vulcanizates were determined, as shown in Fig. 16(a). Both values exhibited the same trend. From the results, both STR 5L and STR 5L loaded with KBM-502 showed the lowest moduli which could be due to their low crosslink densities. In other words, low degree of the network in the rubber compounds resulted in low force resistance of the materials. However, it was found that the moduli of STR 5L blended with G-SSNR were remarkably higher than that of the other two vulcanizates. This could be because of the higher degree of crosslink density, and hence greater compound resistance to deformation. The tensile strength of various vulcanizates was also determined, as presented in Fig. 16(b). This mechanical behavior was also consistent with the modulus. The rubber compound in the presence of G-SSNR showed an improvement in tensile strength. This could be described by the better filler-rubber interaction and silica dispersion which brought about the reinforcing efficiency [31]. On the contrary, STR 5L and STR 5L added with KBM-502 showed the opposite results to STR 5L mixed with G-SSNR, that was because of the inferior filler-rubber interaction and silica dispersion. Large agglomerates of silica in the rubber samples could act as a defect during stretching. For elongation at break, all three samples showed no difference in this kind of mechanical behavior, as shown in Fig. 16(c). The incorporation of KBM-502 and G-SSNR as compatibilizers could not affect the elongation at break of the vulcanizates. Hence, it could be concluded that G-SSNR was the key parameter to improve the modulus and tensile strength, but not the elongation at break of the silica-filled NR vulcanizate. The stress-strain curves of the rubber samples are provided in the supporting information.
Conclusions
KBM-502 as the silane coupling agent was successfully grafted onto the SSNR molecules by UVA-induced reaction. The optimum condition of the grafting reaction was 1 phr of KBM-502 and 5 phr of BPO under 8 min of UVA irradiation time. Additionally, the incorporation of G-SSNR into the rubber compound enhanced the cure characteristics, rubber-filler interaction, silica dispersion and mechanical properties of the compounds. Thus, it can be concluded that the G-SSNR produced from the grafting reaction between SSNR and the silane coupling agent under UVA irradiation, was a potential material for use to increase the compatibility in the silica-filled NR compounds.
References
Choi S-S, Nah C, Jo B-W (2003) Properties of natural rubber composites reinforced with silica or carbon black: influence of cure accelerator content and filler dispersion. Polym Int 52(8):1382–1389. https://doi.org/10.1002/pi.1232
Leblanc JL (2002) Rubber–filler interactions and rheological properties in filled compounds. Prog Polym Sci 27(4):627–687
Prasertsri S, Rattanasom N (2012) Fumed and precipitated silica reinforced natural rubber composites prepared from latex system: mechanical and dynamic properties. Polym Test 31(5):593–605. https://doi.org/10.1016/j.polymertesting.2012.03.003
Choi S-S (2002) Influence of storage time and temperature and silane coupling agent on bound rubber formation in filled styrene–butadiene rubber compounds. Polym Test 21(2):201–208. https://doi.org/10.1016/S0142-9418(01)00071-X
Murakami K, Iio S, Ikeda Y, Ito H, Tosaka M, Kohjiya S (2003) Effect of silane-coupling agent on natural rubber filled with silica generated in situ. J Mater Sci 38(7):1447–1455. https://doi.org/10.1023/a:1022908211748
Sengloyluan K, Sahakaro K, Dierkes WK, Noordermeer JWM (2014) Silica-reinforced tire tread compounds compatibilized by using epoxidized natural rubber. Eur Polym J 51:69–79. https://doi.org/10.1016/j.eurpolymj.2013.12.010
Seo G, Park SM, Ha K, Choi KT, Hong CK, Kaang S (2010) Effectively reinforcing roles of the networked silica prepared using 3,3′-bis(triethoxysilylpropyl)tetrasulfide in the physical properties of SBR compounds. J Mater Sci 45(7):1897–1903. https://doi.org/10.1007/s10853-009-4175-3
Sae-oui P, Sirisinha C, Hatthapanit K, Thepsuwan U (2005) Comparison of reinforcing efficiency between Si-69 and Si-264 in an efficient vulcanization system. Polym Test 24(4):439–446. https://doi.org/10.1016/j.polymertesting.2005.01.008
Sae-oui P, Thepsuwan U, Hatthapanit K (2004) Effect of curing system on reinforcing efficiency of silane coupling agent. Polym Test 23(4):397–403. https://doi.org/10.1016/j.polymertesting.2003.10.002
Kim K-J, White JL (2002) Silica surface modification using different aliphatic chain length silane coupling agents and their effects on silica agglomerate size and processability. Compos Interfaces 9(6):541–556. https://doi.org/10.1163/15685540260494119
Theppradit T, Prasassarakich P, Poompradub S (2014) Surface modification of silica particles and its effects on cure and mechanical properties of the natural rubber composites. Mater Chem Phys 148(3):940–948. https://doi.org/10.1016/j.matchemphys.2014.09.003
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26(1):62–69
Sahakaro K, Beraheng S (2008) Reinforcement of maleated natural rubber by precipitated silica. J Appl Polym Sci 109(6):3839–3848. https://doi.org/10.1002/app.28483
Kawahara S, Kakubo T, Nishiyama N, Tanaka Y, Isono Y, Sakdapipanich JT (2000) Crystallization behavior and strength of natural rubber: skim rubber, deproteinized natural rubber, and pale crepe. J Appl Polym Sci 78(8):1510–1516. https://doi.org/10.1002/1097-4628(20001121)78:8<1510::AID-APP70>3.0.CO;2-4
Sakdapipanich JT, Nawamawat K, Kawahara S (2002) Characterization of the large and small rubber particles in fresh Hevea latex. Rubber Chem Technol 75(2):179–185. https://doi.org/10.5254/1.3544971
Anancharungsuk W, Tanpantree S, Sruanganurak A, Tangboriboonrat P (2007) Surface modification of natural rubber film by UV-induced graft copolymerization with methyl methacrylate. J Appl Polym Sci 104(4):2270–2276. https://doi.org/10.1002/app.25661
Hoven VP, Chombanpaew K, Iwasaki Y, Tasakorn P (2009) Improving blood compatibility of natural rubber by UV-induced graft polymerization of hydrophilic monomers. J Appl Polym Sci 112(1):208–217. https://doi.org/10.1002/app.29408
Nakason C, Kaesaman A, Yimwan N (2003) Preparation of graft copolymers from deproteinized and high ammonia concentrated natural rubber latices with methyl methacrylate. J Appl Polym Sci 87(1):68–75. https://doi.org/10.1002/app.11671
Amnuaypornsri S, Sakdapipanich J, Tanaka Y (2010) Highly purified natural rubber by saponificaion of latex: analysis of green and cured properties. J Appl Polym Sci 118(6):3524–3531. https://doi.org/10.1002/app.32699
Kongkaew C, Dokkhan C, Pattanawanidchai S, Chaikumpollert O, Loykulnant S (2012) Factors affecting creaming efficiency of bio-based polymers, vulcanization and mechanical properties of creamed skim rubber. Biomass Bioenergy 46:233–241. https://doi.org/10.1016/j.biombioe.2012.08.021
Saelao J, Phinyocheep P (2005) Influence of styrene on grafting efficiency of maleic anhydride onto natural rubber. J Appl Polym Sci 95(1):28–38. https://doi.org/10.1002/app.20810
Tanaka Y (1989) Structure and biosynthesis mechanism of natural polyisoprene. Prog Polym Sci 14(3):339–371
Kochthongrasamee T, Prasassarakich P, Kiatkamjornwong S (2006) Effects of redox initiator on graft copolymerization of methyl methacrylate onto natural rubber. J Appl Polym Sci 101(4):2587–2601. https://doi.org/10.1002/app.23997
Wongthong P, Nakason C, Pan Q, Rempel GL, Kiatkamjornwong S (2013) Modification of deproteinized natural rubber via grafting polymerization with maleic anhydride. Eur Polym J 49(12):4035–4046. https://doi.org/10.1016/j.eurpolymj.2013.09.009
Kosmalska A, Zaborski M, Ślusarski L (2003) Adsorption of curatives and activity of silica toward elastomers. Macromol Symp 194(1):269–276. https://doi.org/10.1002/masy.200390092
Mullins L, Tobin NR (1957) Theoretical model for the elastic behavior of filler-reinforced vulcanized rubbers. Rubber Chem Technol 30(2):555–571. https://doi.org/10.5254/1.3542705
Nimpaiboon A, Amnuaypornsri S, Sakdapipanich J (2013) Influence of gel content on the physical properties of unfilled and carbon black filled natural rubber vulcanizates. Polym Test 32(6):1135–1144. https://doi.org/10.1016/j.polymertesting.2013.07.003
Saramolee P, Sahakaro K, Lopattananon N, Dierkes WK, Noordermeer JW (2015) Compatibilization of silica-filled natural rubber compounds by combined effects of functionalized low molecular weight rubber and silane. J Elastomers Plast. https://doi.org/10.1177/0095244314568469
Payne AR, Whittaker RE (1971) Low strain dynamic properties of filled rubbers. Rubber Chem Technol 44(2):440–478. https://doi.org/10.5254/1.3547375
Meissner B (1974) Theory of bound rubber. J Appl Polym Sci 18(8):2483–2491. https://doi.org/10.1002/app.1974.070180826
Pattanawanidchai S, Loykulnant S, Sae-oui P, Maneevas N, Sirisinha C (2014) Development of eco-friendly coupling agent for precipitated silica filled natural rubber compounds. Polym Test 34:58–63. https://doi.org/10.1016/j.polymertesting.2014.01.002
Acknowledgements
The authors would like to acknowledge for the financial support from the Thailand Research Fund (TRF) through IRG5780009 and the basic research grant: BRG5780002. The Center of Excellence for Innovation in Chemistry, Commission on Higher Education (PERCH-CIC) is also acknowledged. Sincere appreciation is extended to Thai Rubber Latex Co., Ltd. for kind support the NR latex.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 97 kb)
Rights and permissions
About this article
Cite this article
Dechnarong, N., Nimpaiboon, A., Kumarn, S. et al. Compatibility enhancement of silica and natural rubber compound using UVA-induced silane-grafted saponified skim natural rubber. J Polym Res 25, 17 (2018). https://doi.org/10.1007/s10965-017-1420-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1420-3