Abstract
In this study, three different approaches were applied to obtain thermoplastic polyurethane (TPU) nanocellular foams. The TPU was synthesized with a 4, 4′-methylenebis (phenyl isocyanate) and 1, 4-butanediol (MDI/BD) hard segment system using a pre-polymer method. The three approaches included increasing the hard segment content, adding a graphene nucleation agent, and replacing the soft segments. Although the synthesized TPUs had a different hardness, it was possible to obtain nanocellular structures with all of the methods. The cell structure is not a function of hardness only. Crystallinity affects the cell structure as well. The addition of graphene and replacement of the soft segments were more effective at yielding nanocellular foams. Our best results showed that after adding 0.1 wt% of graphene, the average cell size of the TPU foam decreased to 715 nm, and the cell density was improved to 4.94 × 1011 cells/cm3. The relative density of the foam could be as low as 0.77. This study first reported elastomer-based nanocellular structures with such low relative density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethane (PU) foams play a vital role in the global foam market. They account for more than 50% of the foam market, and the production of PU foams was projected to reach 74.24 billion USD by 2021 [1]. Nevertheless, most PU foams are thermoset and, therefore, are non-recyclable. On the other hand, thermoplastic polyurethane (TPU) is recyclable and possesses excellent engineering characteristics. Because of its high elasticity, TPU is widely used as a thermoplastic elastomer. Currently, TPU accounts for 13% of the market for thermoplastic elastomers, and it has many applications in sporting goods, automobiles, footwear, electronics, and medical devices [2].
TPU is composed of hard segments (diisocyanates and chain extenders), and soft segments (polyols). The physical properties of TPU are determined by the molecular structure of the soft and hard segments. The soft segments provide TPU with low temperature flexibility, while the hard segments provide it with its mechanical strength. Because many possible hard and soft segments, as well as chain extenders, are available, it is theoretically conceivable to produce countless combinations of TPU. The hardness of TPU can be tuned by adjusting the types and ratio of the soft and hard segments, as well as the reaction steps such as the pre-polymer synthesis method [3, 4].
Unlike PU, TPU is not foamed by adding water during the synthesis process; instead, blowing agents or solvents are needed. One possible blowing agent is CO2 because of its green and non-toxic characteristics. Many polymers have been studied for foaming using CO2, and over the years, the processing technology has significantly improved [5]. In the last twenty years, however, relatively few reports have discussed the preparation of TPU foams using CO2. It is known that the solubility of CO2 in the polyols (soft segments) is significantly higher than that in the hard segments [6]. Furthermore, the glass transition temperatures (Tg) of different soft segments differ [7]. Moreover, controlling the TPU chemical structure directly affects its foaming behavior and final foam structure, with a consequent change of the resultant TPU properties. A good example of this is the recent success of expandable TPU midsole [8].
In the late 1990s, researchers from the Institute of Plastics Processing (IKV), Germany studied the feasibility of TPU foam extrusion and measured the solubility of CO2 in TPU [9, 10]. Dai et al. foamed TPU/clay nanocomposites to generate microcellular TPU fibers by extrusion [11], since these materials are capable of combining the potential fibers and foams. The excellent biocompatibility of TPU drew the attention of biomedical engineers. In this respect, Wintermantel and coworkers prepared open porous polyurethane scaffolds by the MuCell® injection molding process [12,13,14,15], as injection molding is one of the most efficient methods to make polymers of complex geometry. Subsequently, Turng and coworkers fabricated foamed TPU scaffolds by injection molding and extensively investigated the processing variables on the foam’s cell structure [16,17,18]. In addition to biomedical applications, Turng and coworkers also studied the effect of additives on the foaming ability, mechanical properties, and flammabilities of injection-molded TPU foam [19,20,21,22,23].
Batch foaming is the original method to prepare CO2-blown foam [24]. This approach can precisely control the foaming pressure and temperature to generate a stable cell structure and is especially useful when the material is limited. Prasad et al. studied the effect of the hardness and processing variables, such as pressure and temperature, and found they all significantly impacted the TPU foam structure [25]. Naguib et al. foamed TPU/carbon nanotube nanocomposites using CO2 and studied the piezoresistive properties of the TPU nanocomposite foams [26, 27]. Our research group foamed commercially available TPU by batch foaming to prepare nanocellular TPU foams [28]. The mechanical properties of the TPU nanocellular foams were also reported [29].
As noted previously, the physical properties of TPU are strongly affected by its chemical structure. However, the effect of the TPU chemical structures on the foam has not been previously reported. Although the structure–property relationship of TPU prepared with different soft and hard segments has been studied extensively [30], very few studies have synthesized TPU for foaming [6, 31, 32]. In one study, Dai et al. synthesized TPU without a chain extender. The CO2-blown microcellular TPU foams showed excellent damping properties [31]. In another study, Ito et al. were the first to examine the effect of the soft segment molecular weight and the hard segment content on the cell structure of TPU foam [6]. They suggested that most of the CO2 was dissolved in the soft segments and that foaming would most likely occur only at these segments. In our study, TPU was synthesized via a pre-polymer method whereby microcellular TPU was obtained by batch foaming. Our results showed that premixing TPU before foaming could significantly affect the cell structure [32].
Nanocellular foam is known to possess high toughness, ultra-low thermal conductivity, and excellent electrical properties [33,34,35]; therefore, it is a highly preferred material and is the focus of many studies. One effective strategy to create polymer nanocellular foams is to use CO2 as a blowing agent because the foam can be produced in large quantities. At present, research on CO2-blown polymer nanocellular foams is in its infancy. The typical strategies for producing polymer nanofoams include using high-Tg engineered polymers, block copolymers, polymer blends, or adding nanoparticles as nucleation agents [35]. Among these methods, the use of block copolymers is of special interest. Yokoyama et al. grafted CO2-philic polymer blocks of low Tg together with polystyrene blocks. By their nature, the size of polymer blocks is in the nanometer range. Since the CO2 solubility and Tg values of these blocks can differ considerably, it is feasible to choose a temperature at which only the low Tg block would foam to give rise to nanocellular foams [36,37,38,39]. A similar scenario can be envisioned in TPU since the soft and hard domain sizes of TPU are approximately 10–20 nm [40] and since the CO2 solubility of the soft segments is significantly higher than the hard segments [6]. In our previous experiments, nanocellular structures were observed by the foaming of commercially available TPU using CO2 [28]. Nevertheless, very few studies have reported the preparation of elastomer-based nanocellular foams.
In the past, our group created TPU nanocellular foams using commercial and synthesized TPUs. To achieve this, nanoparticles such as nanoclays were added as nucleation agents [28], and it was found that the addition of these nanoclays was beneficial for creating nanocellular structures. Prasad et al. showed that the cell structure of TPU foams made by CO2 foaming is related to the hardness of the polymer matrix [25]. However, structural factors and the addition of fillers may also affect the hardness of TPU, and thus its foaming behavior. Using hardness as the only parameter to describe the foaming behavior of TPU is far from enough. Therefore, a thorough understanding of the relationship between the chemical structure of TPU and its foaming behavior/foam structure is needed.
In this study, we investigated the effect that the hard segment content, soft segment type, and addition of filler nanoparticles would have on the properties and foaming behavior of TPU. For this purpose, TPU was synthesized using an MDI/BD system as the hard segment (without solvent), and using a pre-polymer method. In our previous study, nanoclay was used as the nucleation agent to promote cell nucleation and to create nanocellular foam [28, 29]. However, graphene has shown a higher nucleation efficiency than commercially available nucleation agents such as talc and other carbon-based nanoparticles [41], and it may improve the electromagnetic interference shielding effect of polymeric foams. [42, 43]. For this reason, graphene was chosen as the nanoparticle nucleation agent.
Although TPU/graphene nanocomposites have been extensively studied [44], there is very little literature about the foaming of TPU/graphene nanocomposites using CO2. Chen et al. and Liu et al. studied the properties of TPU/graphene foams, but the foam fabrication process involved the use of toxic solvents such as dioxane and dimethylformamide [45, 46]. In this study, graphene was applied as the nucleation agent to investigate the possibility of producing foam materials that possess a nanocellular structure with a high cell density.
Experimental section
Materials
The polymer was synthesized without a solvent, using a pre-polymer method. The hard segment and chain extenders used in this study were MDI and BD, both of which were purchased from Alfa Aesar. The catalyst used was stannous octoate, which was purchased from TMG Industrial Chemical Company LTD.
Sample preparation
The isocyanate, polyol, and chain extender were all pre-dried using a vacuum oven, or vacuum line with an ice bath, to remove moisture. Next, the MDI was heated to 80 °C in a four-neck round bottom flask, and polyol was added to start the reaction. The system was stirred using an agitator at a speed of 500 rpm in a nitrogen environment with a flow rate of 1 L/min.
The pre-polymerization finished when 50% of the isocyanate groups were consumed. The reaction time was determined according to ASTM D-2572, which characterizes the remaining amount of NCO groups in the pre-polymer. After the pre-polymerization, the chain extender and catalyst were added for chain extension and the agitation rate was raised to 1000 rpm. The loading level of the catalyst was controlled at 400 ppm. This agitation rate was maintained until the temperature reached 85 °C. Then, the final product was poured onto a Teflon plate and cured in an oven at 80 °C for 15 h to complete the reaction. In this study, polypropylene glycol (PPG) with a molecular weight of 1000 was used as the standard polyol, and the molar ratio of MDI:PPG:BD = 2.2:1:1 was used as the standard TPU formulation for the baseline (TPU_01) [32]. An additional of MDI was intentionally used as the moisture scavenger to prevent crosslinking. The polyols used in this study were kindly supplied by Coating P. Materials Co., Ltd., Taiwan.
There were three different sets of experiments: (1) increasing the hard segment content, (2) using graphene as the nucleation agent, and (3) changing the soft segments. In the first part of the experiment, the hardness was increased by raising the hard segment content. The molar ratios of MDI to PPG to BD were increased from 2.2:1:1 to 3.2:1:2 and 4.2:1:3. In other words, the hard segment contents in these samples are 37 wt%, 48 wt%, and 56 wt%, respectively; the corresponding samples are termed TPU_01, TPU_02, and TPU_03, respectively. Since the total content of soft and hard segments is 100%, increasing the hard segment content results in a consequent decrease in the soft segment content.
In the second part of the study, graphene was used as the nucleation agent for the standard TPU polymer (MDI:BD:PPG = 2.2:1:1). Thermally reduced graphene (TRG) was synthesized by oxidizing high purity graphite using the Hummer’s method [47]. This oxidized graphite was then thermally reduced in a tube furnace in an argon environment. The high purity graphite was purchased from Bay Carbon Inc., MI, USA [41]. Graphene was added after the pre-polymerization step was completed, and the solution was agitated at 500 rpm for another 3 min. The loading level of graphene was controlled to be 0 wt%, 0.1 wt%, and 1 wt%. The reason for the large change in loading level is due to the fluffy nature of TRG. That is, thermal reduction has been shown to expand the TRG volume by 100–300 times, yielding very low bulk density TRG sheets [48]. Further, it is not easy to control the dosage of graphene since its bulk density is particularly low, especially for such a small difference of 0.2 wt%. In addition, the effect of such a small loading difference may not be easily observed. Therefore, loading levels of only 0.1 and 1 wt% were chosen for our experiments.
Finally, polytetramethylene ether glycol (PTMEG) and poly (1, 4-butylene adipate) PBA of molecular weight 1000 were used to replace PPG to understand the effect of soft segments, while the other procedures remained unchanged. Detailed information of the TPUs studied is provided in Table 1.
Characterization
The cured TPU was cut into pellets and dried at 80 °C for 4 h. Thereafter, it was compounded using a Xplorer microcompounder at 180–220 °C and 50 rpm for 1 min to enhance the distribution of soft and hard segments [49]. The compounding temperature was adjusted to ensure, as much as possible, that TPU samples of different viscosity were compounded using a similar torque.
The molecular weights of the synthesized TPUs were determined using a Waters ACQUITY Advanced Polymer Chromatography System. The standard was polystyrene. Three layers of 2-mm-thick TPU films were stacked together for hardness testing using a Telock GS-709G durometer. The Tg of the synthesized TPU was determined using a Perkin Elmer Jade differential scanning calorimeter (DSC) using the heat-cool-heat method. Five milligrams of TPU were heated from ambient temperature to 200 °C to remove the thermal history. The sample was then cooled to −100 °C and then heated to 200 °C to determine the Tg. Both the heating and cooling rates were maintained at 10 °C/min.
The foam morphology was characterized using a JEOL JSM-6390LV scanning electronic microscope (SEM). Samples were cryo-fractured using liquid nitrogen and sputter coated with gold for observation. The samples containing graphene were cryo-microtomed (Leica Ultracut) to a thickness of 100 nm to observe the dispersion of graphene in TPU. The microstructures of the samples were examined by transmission electronic microscopy (TEM, JEM-2100F).
Preparation of TPU foam
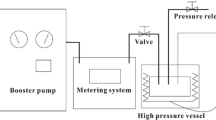
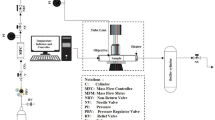
Approximately 0.05 g TPU pellet was cut from the compounded sample to conduct the foaming experiments. The samples were saturated and foamed at various foaming temperatures (Tf) using CO2 in a batch foaming system. In this system, a syringe pump (ISCO-265D) was connected to the foaming cells, via a four way union, to deliver CO2 at a controlled pressure. Three high-pressure inline filters (Swagelok SS-2F-05) were used as the foaming cells. Each foaming cell was connected to a three-way ball valve for pressure release. The foaming cells were immersed in water or oil baths to control the Tf. The foaming process was conducted by quickly opening the three-way ball valve, and the Tf was varied from 20 to 140 °C. The sample saturation time was fixed at 24 h, while the foaming saturation pressure was controlled at 13.79 MPa (2000 psi).
It is difficult to determine the time taken to reach CO2 equilibrium in the samples because of the preferential absorption of CO2 by the soft segments. Additionally, CO2 may cause the polymer to crystallize. The thickness of the foaming sample was assumed to be 2 mm. As CO2 diffused from both sides of the sample, the length of diffusion was approximately 1 mm. A time dependent, one-dimensional diffusion model could be applied in this case. The characteristic length to reach half of the surface (maximum) concentration of the solvent is \( \mathrm{L}=0.475\sqrt{4 Dt} \) [50]. According to research by Ito, Dobashi, and Matsunaga, the diffusivity of CO2 in various TPUs at 15 °C is in the range of 1 × 10−6 cm2/s [6, 7, 51]. Based on a saturation time of 24 h and the literature diffusivity values, the characteristic length to reach half of the surface (maximum) concentration of the solvent was in the range of 5.22 mm. Therefore, 24 h should be sufficient time to ensure that the TPU is saturated.
The density of the samples was measured using Archimedes’ principle. The cell density of the foam was calculated by observing the cryo-fractured cross sectional area of the foam using Eq. (1).
where D is the cell density (cell/cm3); N is the number of cells in the SEM picture; A is the actual measuring area of the SEM picture in cm2; ρp is the density of the polymer; ρf is the density of the foam.
Results and discussion
As mentioned in the Introduction, the hardness of TPU can be modified by increasing the hard segment content, replacing the soft segments, or adding fillers. Our results support these statements, as the physical properties of the synthesized TPU listed in Table 2 indicate. In terms of improving the hardness of TPU, increasing the hard segment content seems to be most effective. Increasing the hard segment content from 37 wt% to 56 wt% changed the hardness significantly. The hardness values of TPU_01, TPU_02, and TPU_03 were 70A, 90A, and 95A, respectively. Second, adding 0.1 wt% and 1 wt% of graphene also increased the hardness. The hardness values of TPU_04 and TPU_05 were 73A and 79A, respectively. Replacing the soft segments of TPU could also change its hardness. The hardness values of PTMEG- and PBA-based TPUs (TPU_06 and TPU_07) were 85A and 90A, respectively. As PPG and PTMEG polyols contain ether groups, the barrier for the rotation of the C–O bond is low [52]. Thus, TPU_01 and TPU_06 were relatively soft. On the other hand, PBA polyol contains ester groups that can form hydrogen bonds with the urethane groups. Therefore, the prepared TPU (TPU_07) was harder than the ether-based TPUs (TPU 01 and TPU_06) [52]. The results are consistent with the results published by Bagdi et al. [53].
It is known that Tg is an important parameter that determines the polymer foaming ability. To this end, DSC was used to determine the Tg values of the synthesized samples, and the results are listed in Table 2. Typical DSC curves of samples with 37, 48, and 56 wt% hard segment content (TPU_01, TPU_02, and TPU_03) are shown in Fig. 1. No apparent melting peak was observed for the samples containing 37 wt% hard segment. This is not surprising. Abouzahr et al. pointed out that for MDI-BD-based TPUs, no apparent crystallization peak was observed when the hard segment content was less than 45 wt% [54]. In addition, for the PPG-based TPU, no significant melting peak was observed when the molecular weight of the soft segment was less than 2000 [3]. Therefore, we can assume that either very few crystals existed in the standard polymer, or the polymer was almost crystal-free. The DSC trace of samples containing 48 and 56 wt% hard segments showed a broad but not significant melting peak. It is known that TPU possesses a broad melting peak [49] and often shows a double melting peak during the heating process. The first melting peak represents the disruption of the long range order in the hard segments, while the second melting peak indicates the melting of the hard segments [4]. There is no melting peak present in TPU_01 as the hard segment content is less than 45 wt%. This is consistent with reference [4] as the hard segment content in TPU_01 was low. The melting temperature of TPU_02 and TPU_03 was around 140–150 °C. The Tg of the PPG-based TPUs (TPU_01 ~ TPU_05) were between −6 – 2 °C. The results are similar to the results from Pattanayak et al. [55,56,57]. On the other hand, the Tg values of PTMEG- and PBA-based TPUs were −29.2 and −13.6 °C, respectively. The Tg mentioned here is the Tg of the soft segments [7, 58].
Details of the characterization of TRG can be found in literature [41]. The surface area of the carbon nanoparticles was determined by Brunauer–Emmett–Teller method (Micromeritics ASAP 2020). The surface area of TRG was 402.02 ± 1.03 m2/g. Adding 0.1 wt% and 1 wt% of graphene increased the hardness of TPU_02 and TPU_03. This was expected as it is known that the addition of graphene increases the mechanical properties of TPU [44]. The addition of graphene also slightly increased the Tg of TPU. The Tg of neat TPU was −1 °C. Adding 0.1 wt% and 1 wt% of graphene increased the Tg of TPU to 1.9 °C and 0.6 °C, respectively. Until now, the effect of graphene and its loading level on Tg has not been very clear. Liao et al. used poly(methyl methacrylate) as a model polymer and carefully examined various methods of preparing polymer/graphene nanocomposites [59]. This work also reviewed the Tg values of polymer graphene nanocomposites reported in the literature up until 2014. Their conclusions indicated that a significant increase in Tg is not expected unless the polymer/graphene nanocomposites are synthesized by in situ polymerization, the polymer matrix is highly polar, or the graphene platelets are surface modified. The Tg values of the TPU/graphene nanocomposites reported in the Supporting Information of that work, as well as the present results, support these conclusions. That is, no significant increase in Tg is observed.
As can be seen in Table 2, TPUs made with different polyols have different Tg values. Among them, the PTMEG-based TPU exhibit the lowest Tg. Usually, ether-based TPUs such as those containing PTMEG and PPG should possess a lower Tg than ester-based TPUs [7, 60]. However, in our case, the PPG-based TPU possesses the highest Tg. The methyl groups on the side chain of PPG and the molecular weight may play a vital role. Increasing the molecular weight of PPG from 1000 to 2000 may lower the Tg by more than 30 °C [3, 55,56,57].
In our previous study, we observed that TPU_01 could be foamed and a uniform cell structure could be obtained as long as Tf was above 20 °C [32]. It is believed that when Tf is higher than the Tg of the polymer, amorphous polymer can be foamed by one step batch foaming. Here, one step foaming means saturating the polymer above its Tg and the polymers were foamed right after pressure release. Indeed, some researchers used these characteristics to determine the Tg, or even sketch the Tg depression line of amorpous polymers [61]. The minimum Tf values of the synthesized TPU are listed in Table 2. Note that the minimum Tf is defined as the Tf that results in a uniform cell structure.
When the hard segment content of the PPG-based TPU (samples TPU_02 and TPU_03) increased, the hard segments of the polymer started to crystallize. As the hardness of TPU_02 and TPU_03 increased to 90A and 95A, the minimum Tf of the sample increased to 100 °C and 140 °C, respectively. For a crystallized polymer, this is not surprising. Bao et al. studied the foaming ability of PP and observed a similar phenomenon. The minimum Tf was much higher than the Tg of PP [62].
Another example was found when the soft segments were changed. The Tg values of PTMEG- and PBA-based TPUs (TPU_06 and TPU_07) were −29.2 °C and −13.6 °C, respectively. As the hard segment content of the TPU_06 and TPU_07 samples was less than 45 wt% and because the molecular weight of the soft segments was less than 1000, there was almost no crystallization in the sample [54]. However, these two samples could not be foamed at a Tf below 60 °C. These results indicate that minimum Tf is also a function of hardness. In the case of foaming elastomers, Tg is not the only factor that determines whether the sample can be foamed. Both hardness and crystallinity significantly affect the foaming capability of the samples.
Since the Tg values and CO2 solubility of the soft and hard segments differ widely, and since the domain size of the soft and hard domains was approximately 10–20 nm [6, 40], it was possible to create nanocellular structures by the batch foaming method. The cell structure of different TPU foams will be discussed in the following sections. First, we will discuss the effect of the content of the hard segments on the cell structure. The cell size and cell density of foamed TPU_01, TPU_02, and TPU_03 are given in Table 3. It is worth noting that the minimum Tf values of TPU_02 and TPU 03 were 100 and 140 °C, respectively; below the minimum Tf, a non-uniform structure was observed.
As can be observed in Table 3, the average cell size generally decreased with Tf. For the TPU_01 sample, the lowest average cell size obtained was approximately 2 μm, and the relative density was between 0.34 and 0.71. Conversely, a sub-micron structure was successfully obtained in the TPU_02 sample. The lowest average cell size obtained was approximately 500–600 nm; however, a non-uniform cell structure was observed in foamed TPU_02. To describe such a phenomenon, if two different cell densities were observed at the same Tf, the two cell densities were recorded. For example, at a Tf of 60 °C, two cell densities of 8 × 1010 and 4 × 109 cells/cm3 were observed. Similarly, the differences in cell sizes are recorded in Table 3. The SEM images of foamed TPU_02 samples of high cell density and low cell density are shown in Fig. 2a and b, respectively. As the Tf was higher than the Tg, but lower than the melting temperature, this structure should be related to the crystal structure. A crystallized polymer usually does not absorb CO2 and cannot be foamed. Further, the cell growth may be confined in between the polymer crystals, and thus, nanocells are created. Similar phenomena were observed by Bao et al. [62] and Jiang et al. [63]. In their case, polypropylene was foamed by batch foaming. The crystallized polymer was not foamed, and the cell structure was not uniform. If the polymers were foamed close to their melting temperature, only the inter-lamella amorphous region would foam, leading to the formation of non-uniform cell structures [63]. It is interesting to note that although the cell structure was non-uniform, the cell sizes were quite close. This is very different from our previous study [32].
When Tf increased to 80 °C, the cell size ranged from 600 to 800 nm. The cell densities of a low cell area and high cell area were 3.1 × 1010 and 1.06 × 1010 cells/cm3, respectively. The foam density of TPU_02 ranged from 0.9–1 g/cm3. The non-uniform cell structure disappeared when Tf reached 100 °C. Although the melting temperature of TPU_02 was approximately 140–150 °C, a very broad melting peak was observed in the DSC trace. This indicates that some of the crystals may have melted at 100 °C. Dissolving CO2 in the polymer may lower the melting temperature of the polymers. It is possible that the crystals were molten and thus created a uniform cell structure. Although the crystallinity of our samples was not reported, the crystallinity of the MDI-based TPU is often ~20% [30]. Such a low crystallinity may cause the minimum Tf to be lower than the melting temperature.
On the other hand, a non-uniform cell structure was observed again in TPU_03. It cannot be foamed below 100 °C. In particular, a nanocellular structure was not observed. Similar to the TPU_01 and TPU_02 samples, the cell density was also of the order of 1010 cells/cm3. The cell structure became uniform at 140 °C, but the cell size decreased to 1.1 μm. Additionally, the relative density increased to 0.89. As 140 °C is close to the melting temperature of TPU_03 (see Fig. 1), the cell may shrink or collapse during the foaming process and cause cell shrinkage.
Since the hard segment content of TPU_03 was well above 45 wt%, it would definitely crystallize [54], and its crystallinity would be higher than TPU_02. Doroudiani et al. foamed several different semi-crystalline polymers of different crystallinity and found that crystallinity has a strong influence on the foamed product. Specifically, as the crystallinity increased, the cell structure first became non-uniform and then the polymers became incapable of foaming [64]. To overcome the increase in crystallinity, the value of Tf must be elevated. We believe this is why the minimum Tf of TPU_03 is significantly higher than that of TPU_02. A similar observation was noted in a one-step batch foaming of polypropylene, where the difference between the minimum Tf and the melting temperature was found to increase with the CO2 saturation pressure [65]. Although the resultant crystallinity was not reported, it is known that high saturation pressure increases the PP crystallinity [66].
The cell size and cell density of foamed TPU samples reinforced with 0.1 and 1 wt% graphene are given in Table 4. Tf ranged from 20 to 80 °C. The cell size decreased, but the cell density increased with decreasing Tf. When Tf reached 20 °C, a nanocellular structure was observed. Adding 0.1 wt% of graphene decreased the cell size from 1.46 μm to 715 nm. In addition, the cell density increased from 1.06 × 1011 cells/cm3 to 4.94 × 1011 cells/cm3. The relative density of the sample containing 0.1 wt% graphene (TPU_04) was 0.77. It is worth noting that in our previous studies, when the cell size reached a submicron level, the relative density of the TPU foam was as high as 0.94 [28]. The results showed that adding 0.1 wt% graphene was very helpful in promoting cell nucleation, which in turn reduced the foam density. Such exciting results encouraged us to further increase the graphene loading level to 1 wt%. However, the cell density did not increase as expected, and the cell size was increased. We suspect this result is related to the poor dispersion of graphene in TPU.
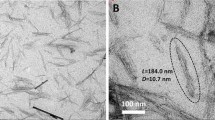
The TEM images of samples containing 0.1 and 1 wt% of graphene are shown in Fig. 3. In these images, the black lines indicate graphene. As can be seen from Fig. 3, there are many graphene aggregates in the samples containing 1 wt% graphene. On the other hand, although fewer aggregates can be observed in the sample containing 0.1 wt% graphene, there were no exfoliated graphene platelets. When the graphene loading level increased to 1 wt%, the cell size increased significantly, and the cell density decreased. The results are consistent with the TEM images. This clearly indicates that poor nanoparticle dispersion may result in a poor cell structure. Similar results were reported before in PMMA-carbon nanotube nanocomposite foams [67].
As seen in Table 2, adding graphene significantly increased the hardness of the foams. The foaming results also showed that adding only 0.1 wt% graphene significantly decreased the cell size and increased the cell density of the foam. However, when the graphene loading level increased to 1 wt%, the cell size did not further decrease, and the cell density increased. This result, however, also indicates a good dispersion of graphene at a loading of 0.1 wt% in the TPU matrix, and the results were confirmed by the TEM images.
The soft segments of TPU_06 and TPU_07 are PTMEG and PBA, respectively. Table 5 lists the relative density, cell size, and cell density of the foamed samples. The relative density increased with decreasing Tf. Because the cell growth rate is inversely proportional to viscosity [68, 69] and since viscosity decreases with temperature, the cell size usually increases with Tf [28, 70,71,72]. The low viscosity resulted in less resistance to cell growth, which could result in a large cell size and low foam density.
The cell size decreased as Tf decreased. At a Tf of 60 °C, the cell sizes of TPU_01, TPU_06, and TPU_07 were 1.86 μm, 0.77 μm, and 0.69 μm, respectively. The hardness of these samples increased from 70A to 90A, while the cell size decreased by 63%. It is worthy to note that the TPU_06 and TPU_07 were not able to foam at a Tf below 60 °C. No cells were observed. Thus, we can say that the minumum Tf of TPU_06 and TPU_07 was approximately 60 °C. These results are very interesting as they indicate that both crystallinity and hardness can affect the minimum Tf and that crystallinity plays a more critical role in this process. Overall, since research on foaming thermoplastic elastomers is in its very early stages, these results provide valuable information for both academic- and industry-driven research. In the near future, a more detailed follow up study on role of foaming pressure, different additives, and crystallinity will be performed by our group.
An interesting question is whether the density of the TPU nanocell foam can be further reduced. In our previous results, when the cell size was less than 1 μm, the relative density was higher than 0.9. If the cell density can be increased, the relative density may be further reduced. For some polymers, it is possible to generate a nanocellular foam with a cell density of 1012–1015 cells/cm3 [73, 74]. Nevertheless, the results in this study and our previous studies showed that the cell density of TPU-based nanocellular foams is approximately 1010–1011 cells/cm3 [28].
It is known that increasing the solubility of the blowing agent may increase the cell density [75]. To observe the effect of increasing the solubility of the blowing agent on the cell density, commercial available TPUs were foamed at 27.58 MPa (4000 psi) and 50–80 °C by a one-step batch foaming process. Nevertheless, the cell density was still in the order of 1011 cells/cm3. These results can be found elsewhere [76]. After a thorough literature survey, an interesting result was found. Even though previous studies used different TPUs, all of them reported the upper limit of the cell density of TPU foams to be approximately 1011 cells/cm3 [6, 31, 32, 49]. It appears that the upper limit of the cell density of TPU foams is of the order of 1011 cells/cm3.
In summary, the best cell morphologies obtained using the three approaches are presented in Table 6. The results show that adding 0.1 wt% graphene may provide the best cell morphology. The SEM images of adding 0.1 wt% graphene (TPU_04) and changing soft segments (TPU_07) is shown in Fig. 4. Clear differences are observed. Adding 0.1 wt% graphene created much higher cell densities.
Conclusions
In this study, TPU was prepared from an MDI-BD system using the pre-polymer method, and the foamed material was prepared by batch foaming. Three approaches were adopted to prepare TPU nanocellular materials. The first method involved increasing the TPU hard segment content to create hard segment crystals and observe if these crystals confined the cells during the cell growth or induced hetrogeneous nucleation. The second method required the addition of a nucleation agent. Nanoparticles (graphene) were added as the nucleation agent. The last method was to control the hardness of different TPUs by using different soft segments. All three methods were capable of producing cell sizes of less than 1 μm. Although increasing the hard segment content resulted in a minimum average cell diameter of 500 nm, the cell size distribution was nonuniform. A bimodal cell structure was observed, and the relative density was as high as 0.91. The addition of nanoparticles as nucleation agents was effective than increasing the hard segment content or replacing the soft segments. Both methods yileded a homogeneous cell structure with a cell size of approximately 700 nm. Replacing the soft segments only resulted in a relative density of 0.9. Adding only 0.1 wt% graphene was the better choice as the relative density could reach a value of 0.77.
In addition to producing a nanocellular strucutre, our results also provided new insights into solid-state foaming. In the solid-state foaming of TPU, whether the sample would be foamed is not determined by its Tg alone. The hardness and crystallinity also played an important role. According to the findings of this study, the harder the polymer and the higher its crystallinity, the higher is the minimum Tf, and it is much higher than the Tg of TPU. We suspect that the same concept may be applied to the foaming of other elastomers.
References
Polyurethane Foam Market worth 74.24 Billion USD by 2021. http://www.marketsandmarkets.com/PressReleases/polyurethane-foams.asp Accessed 11 Sept 2017
Oertel GN, Abele L (1994) Polyurethane handbook : chemistry, raw materials, processing, application, properties2nd edn. Hanser, New York
Pattanayak A, Jana SC (2005) Thermoplastic polyurethane nanocomposites of reactive silicate clays: effects of soft segments on properties. Polymer 46(14):5183–5193
Tien YI, Wei KH (2000) Thermal transitions of montmorillonite/polyurethane nanocomposites. J Polym Res 7(4):245–250
Tomasko DL, Burley A, Feng L, Yeh SK, Miyazono K, Nirmal-Kumar S, Kusaka I, Koelling K (2009) Development of CO2 for polymer foam applications. J Supercrit Fluids 47(3):493–499
Ito S, Matsunaga K, Tajima M, Yoshida Y (2007) Generation of microcellular polyurethane with supercritical carbon dioxide. J Appl Polym Sci 106(6):3581–3586
Matsunaga K, Sato K, Tajima M, Yoshida Y (2005) Gas permeability of thermoplastic polyurethane elastomers. Polym J 37(6):413–417
Prissok F, Braun F (2012) Foams based on thermoplastic polyurethanes. US Patent 20100222442
Kropp D, Michaeli W, Herrmann T, Schröder O (1998) Foam extrusion of thermoplastic elastomers using CO2 as blowing agent. J Cell Plast 34(4):304–311
Michaeli W, Heinz R (2000) Foam extrusion of thermoplastic polyurethanes (TPU) using CO2 as a blowing agent. Macromol Mater Eng 284-285(1):35–39
Dai C, Zhang C, Huang W, Chang K-C, Lee LJ (2013) Thermoplastic polyurethane microcellular fibers via supercritical carbon dioxide based extrusion foaming. Polym Eng Sci 53(11):2360–2369
Leicher S, Will J, Haugen H, Wintermantel E (2005) MuCell® technology for injection molding: a processing method for polyether-urethane scaffolds. J Mater Sci 40(17):4613–4618
Haugen H, Will J, Fuchs W, Wintermantel E (2006) A novel processing method for injection-molded polyether-urethane scaffolds. Part 1: processing. J Biomed Mater Res B Appl Biomater 77(1):65–72
Wu H, Wintermantel E, Haugen HJ (2010) The effects of mold design on the pore morphology of polymers produced with MuCell ®Technology. J Cell Plast 46(6):519–530
HB W, Haugen HJ, Wintermantel E (2012) Supercritical CO2 in injection molding can produce open porous polyurethane scaffolds - a parameter study. J Cell Plast 48(2):141–159
Mi HY, Jing X, Peng J, Turng LS, Peng XF (2013) Influence and prediction of processing parameters on the properties of microcellular injection molded thermoplastic polyurethane based on an orthogonal array test. J Cell Plast 49(5):439–458
Mi H-Y, Jing X, Salick MR, Crone WC, Peng X-F, Turng L-S (2014) Approach to fabricating thermoplastic polyurethane blends and foams with tunable properties by twin-screw extrusion and microcellular injection molding. Adv Polym Technol 33(1):21380
Mi H-Y, Jing X, Salick MR, Peng X-F, Turng L-S (2014) A novel thermoplastic polyurethane scaffold fabrication method based on injection foaming with water and supercritical carbon dioxide as coblowing agents. Polym Eng Sci 54(12):2947–2957
Sun X, Kharbas H, Turng L-S (2015) Fabrication of highly expanded thermoplastic polyurethane foams using microcellular injection molding and gas-laden pellets. Polym Eng Sci 55(11):2643–2652
Wang X-C, Jing X, Peng Y-Y, Ma Z-K, Liu C-T, Turng L-S, Shen C-Y (2016) The effect of nanoclay on the crystallization behavior, microcellular structure, and mechanical properties of thermoplastic polyurethane nanocomposite foams. Polym Eng Sci 56(3):319–327
Kharbas HA, Ellingham T, Manitiu M, Scholz G, Turng LS (2017) Effect of a cross-linking agent on the foamability of microcellular injection molded thermoplastic polyurethane. J Cell Plast 53(4):407–423
Kharbas HA, McNulty JD, Ellingham T, Thompson C, Manitiu M, Scholz G, Turng LS (2017) Comparative study of chemical and physical foaming methods for injection-molded thermoplastic polyurethane. J Cell Plast 53(4):373–388
Wang X-C, Geng T, Han J, Liu C-T, Shen C-Y, Turng L-S, Yang HE (2017) Effects of nanoclays on the thermal stability and flame retardancy of microcellular thermoplastic polyurethane nanocomposites. Polym Compos. https://doi.org/10.1002/pc.24340
Martini-Vvedensky JE, Sue NP, Waldman FA (1984) Microcellular closed cell foams and their method of manufacture. US Patent Number US 4473665
Prasad A, Fotou G, Li S (2013) The effect of polymer hardness, pore size, and porosity on the performance of thermoplastic polyurethane-based chemical mechanical polishing pads. J Mater Res 28(17):2380–2393
Rizvi R, Naguib H (2013) Porosity and composition dependence on electrical and piezoresistive properties of thermoplastic polyurethane nanocomposites. J Mater Res 28(17):2415–2425
Ugalde AR, Naguib HE (2017) Double-layer membrane cathode with improved oxygen diffusivity in zinc-air batteries. Energy Storage Mater 8:1–9
Yeh S-K, Liu Y-C, Wu W-Z, Chang K-C, Guo W-J, Wang S-F (2013) Thermoplastic polyurethane/clay nanocomposite foam made by batch foaming. J Cell Plast 49(2):119–130
Yeh S-K, Liu Y-C, Chu C-C, Chang K-C, Wang S-F (2017) Mechanical properties of microcellular and Nanocellular thermoplastic polyurethane nanocomposite foams created using supercritical carbon dioxide. Ind Eng Chem Res 56(30):8499–8507
Gaymans RJ (2011) Segmented copolymers with monodisperse crystallizable hard segments: novel semi-crystalline materials. Prog Polym Sci 36(6):713–748
Dai X, Liu Z, Wang Y, Yang G, Xu J, Han B (2005) High damping property of microcellular polymer prepared by friendly environmental approach. J Supercrit Fluids 33(3):259–267
Chu CC, Yeh SK, Peng SP, Kang TW, Guo WJ, Yang J (2017) Preparation of microporous thermoplastic polyurethane by low-temperature supercritical CO2 foaming. J Cell Plast 53(2):135–150
Notario B, Pinto J, Rodriguez-Perez MA (2016) Nanoporous polymeric materials: a new class of materials with enhanced properties. Prog Mater Sci 78-79:93–139
Forest C, Chaumont P, Cassagnau P, Swoboda B, Sonntag P (2015) Polymer nano-foams for insulating applications prepared from CO2 foaming. Prog Polym Sci 41:122–145
Costeux S (2014) CO2-blown nanocellular foams. J Appl Polym Sci 131(23):41293
Li L, Yokoyama H, Nemoto T, Sugiyama K (2004) Facile fabrication of Nanocellular block copolymer thin films using supercritical carbon dioxide. Adv Mater 16(14):1226–1229
Yokoyama BH, Li L, Nemoto T, Sugiyama K (2004) Tunable Nanocellular polymeric monoliths using fluorinated block copolymer templates and supercritical carbon dioxide. Adv Mater 16(17):1542–1546
Yokoyama H, Sugiyama K (2005) Nanocellular structures in block copolymers with CO2-philic blocks using CO2 as a blowing agent: crossover from micro- to Nanocellular structures with depressurization temperature. Macromolecules 38(25):10516–10522
Li L, Nemoto T, Sugiyama K, Yokoyama H (2006) CO2 foaming in thin films of block copolymer containing fluorinated blocks. Macromolecules 39(14):4746–4755
Qi HJ, Boyce MC (2005) Stress–strain behavior of thermoplastic polyurethanes. Mech Mater 37(8):817–839
Yeh SK, Ke MQ, Cheng KC, Guo WJ, Chung RJ (2013) Effects of different carbon nanoparticles to the morphology of PS/carbon nanocomposite foam. In: Annual Technical Conference - ANTEC, Conference Proceedings, pp 360–364
Zhang HB, Yan Q, Zheng WG, He ZX, Yu ZZ (2011) Tough graphene-polymer microcellular foams for electromagnetic interference shielding. ACS Appl Mater Interfaces 3(3):918–924
Antunes M, Velasco JI (2014) Multifunctional polymer foams with carbon nanoparticles. Prog Polym Sci 39(3):486–509
Kim H, Miura Y, Macosko CW (2010) Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem Mater 22(11):3441–3450
Chen Y, Li Y, Xu D, Zhai W (2015) Fabrication of stretchable, flexible conductive thermoplastic polyurethane/graphene composites via foaming. RSC Adv 5(100):82034–82041
Liu H, Dong M, Huang W, Gao J, Dai K, Guo J, Zheng G, Liu C, Shen C, Guo Z (2017) Lightweight conductive graphene/thermoplastic polyurethane foams with ultrahigh compressibility for piezoresistive sensing. J Mater Chem C 5(1):73–83
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339
Kim H, Abdala AA, Macosko CW (2010) Graphene/polymer nanocomposites. Macromolecules 43(16):6515–6530
Hossieny NJ, Barzegari MR, Nofar M, Mahmood SH, Park CB (2014) Crystallization of hard segment domains with the presence of butane for microcellular thermoplastic polyurethane foams. Polymer 55(2):651–662
Bird RB, Stewart WE, Lightfoot EN (2002) Transport phenomena2nd edn. Wiley, New York
Dobashi R, Matsunaga K, Tajima M (2014) Effects of fullerene derivatives on the gas permeability of thermoplastic polyurethane elastomers. J Appl Polym Sci 131(6):39986
Ravve A (2012) Principles of polymer chemistry. Springer, New York
Bagdi K, Molnar K, Sajo I, Pukanszky B (2011) Specific interactions, structure and properties in segmented polyurethane elastomers. Express Polym Lett 5(5):417–427
Abouzahr S, Wilkes GL (1984) Structure property studies of polyester- and polyether-based MDI–BD segmented polyurethanes: effect of one- vs. two-stage polymerization conditions. J Appl Polym Sci 29(9):2695–2711
Pattanayak A, Jana SC (2005) High-strength and low-stiffness composites of nanoclay-filled thermoplastic polyurethanes. Polym Eng Sci 45(11):1532–1539
Pattanayak A, Jana SC (2005) Properties of bulk-polymerized thermoplastic polyurethane nanocomposites. Polymer 46(10):3394–3406
Pattanayak A, Jana SC (2005) Synthesis of thermoplastic polyurethane nanocomposites of reactive nanoclay by bulk polymerization methods. Polymer 46(10):3275–3288
Lem K, Haw J, Curran S, Sund S, Brumlik C, Song G, Lee D (2013) Effect of hard segment molecular weight on concentrated solution and bulk properties of ether based thermoplastic polyurethanes. Nanosci Nanoeng 1(1):71–88
Liao K-H, Aoyama S, Abdala AA, Macosko C (2014) Does graphene change Tg of nanocomposites? Macromolecules 47(23):8311–8319
Bagdi K, Molnár K, Kállay M, Schön P, Vancsó JG, Pukánszky B (2012) Quantitative estimation of the strength of specific interactions in polyurethane elastomers, and their effect on structure and properties. Eur Polym J 48(11):1854–1865
Weller JE (1996) The effects of processing and microstructure on the tensile behavior of microcellular foams. Ph.D. dissertation, Department of Mechanical Engineering, University of Washington, USA
Bao JB, Liu T, Zhao L, Barth D, Hu GH (2011) Supercritical carbon dioxide induced foaming of highly oriented isotactic polypropylene. Ind Eng Chem Res 50(23):13387–13395
Jiang XL, Liu T, Xu ZM, Zhao L, Hu GH, Yuan WK (2009) Effects of crystal structure on the foaming of isotactic polypropylene using supercritical carbon dioxide as a foaming agent. J Supercrit Fluids 48(2):167–175
Doroudiani S, Park CB, Kortschot MT (1996) Effect of the crystallinity and morphology on the microcellular foam structure of semicrystalline polymers. Polym Eng Sci 36(21):2645–2662
Xu ZM, Jiang XL, Liu T, Hu GH, Zhao L, Zhu ZN, Yuan WK (2007) Foaming of polypropylene with supercritical carbon dioxide. J Supercrit Fluids 41(2):299–310
Lei Z, Ohyabu H, Sato Y, Inomata H, Smith RL (2007) Solubility, swelling degree and crystallinity of carbon dioxide–polypropylene system. J Supercrit Fluids 40(3):452–461
Zeng C, Hossieny N, Zhang C, Wang B, Walsh SM (2013) Morphology and tensile properties of PMMA carbon nanotubes nanocomposites and nanocomposites foams. Compos Sci Technol 82:29–37
Khan I, Adrian D, Costeux S (2015) A model to predict the cell density and cell size distribution in nano-cellular foams. Chem Eng Sci 138:634–645
Taki K (2008) Experimental and numerical studies on the effects of pressure release rate on number density of bubbles and bubble growth in a polymeric foaming process. Chem Eng Sci 63(14):3643–3653
Barlow C, Kumar V, Flinn B, Bordia RK, Weller J (2001) Impact strength of high density solid-state microcellular polycarbonate foams. J Eng Mater Technol 123(2):229–233
Guo H, Nicolae A, Kumar V (2015) Solid-state poly(methyl methacrylate) (PMMA) nanofoams. Part II: low-temperature solid-state process space using CO2 and the resulting morphologies. Polymer 70:231–241
Urbanczyk L, Calberg C, Detrembleur C, Jérôme C, Alexandre M (2010) Batch foaming of SAN/clay nanocomposites with scCO2: a very tunable way of controlling the cellular morphology. Polymer 51(15):3520–3531
Guo H, Nicolae A, Kumar V (2015) Solid-state microcellular and nanocellular polysulfone foams. J Polym Sci B Polym Phys 53(14):975–985
Miller D, Chatchaisucha P, Kumar V (2009) Microcellular and nanocellular solid-state polyetherimide (PEI) foams using sub-critical carbon dioxide I. Processing and structure. Polymer 50(23):5576–5584
Krause B, Sijbesma HJP, Munuklu P, van der Vegt NFA, Wessling M (2001) Bicontinuous nanoporous polymers by carbon dioxide foaming. Macromolecules 34(25):8792–8801
Liu YC (2012) Production of nanoporous thermoplastic polyurethane nanocomposites by supercritical carbon dioxide. Master Thesis, Department of Chemical Engineering and Biotechnology, National Taipei University of Technology, Taiwan
Acknowledgements
The author would like to thank the Ministry of Science and Technology of Taiwan for funding this research. This work was funded by contract number NSC 102-2221-E-027-109-MY3 and MOST-105-2221-E-011-158 -.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeh, SK., Chen, YR., Kang, TW. et al. Different approaches for creating nanocellular TPU foams by supercritical CO2 foaming. J Polym Res 25, 30 (2018). https://doi.org/10.1007/s10965-017-1419-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1419-9