Abstract
Thermoplastic polyurethane (TPU)/olefin block copolymer (OBC)/polycaprolactone (PCL) blends (70/20/10 and 50/30/20) were melt-blended to form the first environmental OBC-based triple-shape memory polymer blends. In this work, PCL with low crystalline temperature (switching phase), OBC with medium crystalline temperature (switching phase), and TPU with high crystalline temperature (fixed phase) could form an alternative triple-shape memory polymer (TSMP). Two compatibilizers, OBC-g-glycidyl methacrylate (OBC-g-GMA) and dicumyl peroxide, were confirmed to show a synergistic effect in enhancing the compatibility further through the morphological observation. Crystallinity of both OBC and PCL in the blends with or without modification decreased in comparison with that of pure resin. For dual-shape behaviors, the shape fixing ratio (Rf) and shape recovery ratio (Rr) were up to 96.3% and 91.2% for the GMA and peroxide-modified blends (50/30/20). The higher amount of TPU didn’t give higher recovery ratio, but instead slightly lower Rr due to the morphology difference. For triple-shape behaviors, both TPU/OBC/PCL blend compositions with or without GMA or peroxide modifications gave high Rf(C→B) values in the first fixing stage, but slightly lower values Rf(B→A) in the second fixing stage, especially for (70/20/10) case. On the other hand, a reverse trend was observed for two recovery stages. To enhance the Rf(B→A) in the second fixing stage, higher deformation temperatures were considered, and a measurable increment on Rf(B→A) was attained. Through this subtle adjustment on the temperature difference between high and low deformation temperatures, the theoretical multi-shape memory shape could be readily tailored to meet different applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Smart polymers or intelligent polymers being capable of instantly responding to environmental stimulations have received much attention recently in light of biomimic concept. Among those polymers, shape memory polymers (SMPs) show interesting shape memory behaviors in the course of developing novel smart materials. SMPs were often deformed at some deformation temperatures to fix their temporary shapes first, which could be restored to their permanent shapes under different stimulus sources, such as heat, light, pH, electric, and magnetic fields. Several potential applications of SMPs such as biomedical, textile, and aerospace sectors were briefly reviewed in the literature [1,2,3,4].
Among those earlier developed materials in the biomedical applications, such as polynorbornene and thermoplastic polyurethane (TPU) [1, 2], TPU with segmented blocks composed of hard blocks and soft blocks often offered a wide range of shape memory effects (SME) due to its richness in different structures. In general, the shape memory programming involved the shape fixing process and recovery process. At first, the sample was deformed at the temperature above its glass transition temperature, crystalline temperature, or other transition temperatures (the switching temperature, Ts) through the softening of soft blocks, and subsequently the deformed shape was temporarily fixed while cooling to room temperature. And then, the temporarily fixed sample could be heated again above the switching temperature to regain its shape via the elastomeric effect from the hard blocks (fixed phase) serving as physical crosslinks. In addition to the conventional thermoplastic elastomers like TPU, one novel thermoplastic elastomer, OBC (olefin block copolymer) recently developed by Dow Chemical Co. also showed unique feature of segmented blocks comprising crystallizable ethylene-octene blocks with high melting temperature (hard phase) and amorphous ethylene-octene blocks (soft phase) with low glass transition temperature [5]. On behalf of unique nanocrystalline hard domains, OBC not only exhibited high melting temperature, but also remained an elastomeric feature in comparison with general olefin-based elastomers with relatively low melting temperature. To our best knowledge, only three articles involved OBC-based SMPs (OBC/paraffin and OBC/hexadecane blends) were available in the literature up to date [6,7,8].
Besides dual-shape memory polymers through the contribution of switching phase and fixed phase as mentioned earlier, several novel SMPs were developed to expand their scopes. Among them, triple-shape memory polymers with additional transition temperature added versatile values for dual-shape memory polymers. Owing to this special feature, triple-shape polymers were not developed till recently [9,10,11,12,13,14,15,16]. However, most works focused on the complex synthesis approaches, and only limited works have focused on the development of triple-shape memory polymer (TSMP) blends using a simple melt-blending and crosslinking process [17,18,19,20,21,22,23]. Hoeher et al. [20] investigated the possibility of tuning triple-shapes for a lightly cross-linked polyethylene SMP blends comprising 80 wt% ethylene olefin copolymer (EOC), 15 wt% linear density polyethylene (LDPE), and 5 wt% high density polyethylene (HDPE) with relatively high strain storage capacity. Unfortunately, these blends were completely crosslinked, which inevitably encountered the problem of recycling issue. Thus, it is our attempt to develop the novel TSMP blends without substantial crosslinking via a melt-blending process in consideration of increasing environmental awareness for sustainable society. To the authors’ knowledge, very rare work prepared the triple-shape memory polymer eco-blend via a simple melt-blending without a fully crosslinked network up to date [23,24,25,26]. In particular, our work is the first environmental OBC-based triple-shape memory polymer blends, especially in combination with biobased polymers like both TPU and polycaprolactone (PCL). With a suitable adjustment on the composition and shape memory programming of TPU/OBC/PCL blends (70/20/10 and 50/30/20), the multi-shape memory effect could be tailored. In this work, PCL with low crystalline temperature (switching phase), OBC with medium crystalline temperature (switching phase), and TPU with high crystalline temperature (fixed phase) could provide an alternative TSMP with eco-concept for the sake of our environment. To improve the compatibility for the TSMP blends, two compatibilizers, OBC-g-glycidyl methacrylate (OBC-g-GMA) and dicumyl peroxide, were used to compare with the uncompatibilized blends. Through this compatibilization technique, the prepared blends with GMA and peroxide modification gave the highest shape recovery ratio more than 90% in the course of dual-shape development. In addition, two shape memory programming processes were implemented to create dual-shape and triple-shape memory blends, respectively. Most of all, through this subtle adjustment on the temperature difference between high and low deformation temperatures, the theoretical multi-shape memory shape could be readily tailored to meet different applications.
Experimental
Materials
The materials used in this study were thermoplastic polyurethane (TPU), olefin block copolymer (OBC), and poly(ε-caprolactone) (PCL). TPU with the trade name 8785A DPS101 was purchased from Bayer, Leverkusen, Germany. OBC (Infuse 9100) with the melt flow index of 1.0 g/10 min (190 °C, 2.16 kg) was supplied by Dow Chemical, Michigan, USA. PCL with the melt flow index of 3.0 g/10 min (160 °C, 2.16 kg) was produced by Perstorp, Perstorp, Sweden and under the trade name of Capa 6800. Dicumyl peroxide (DCP, First Chemicals Co., Ltd., Taipei, Taiwan) was the reagent grade and was used to as an initiator and a compatibilizer. Glycidyl methacrylate (GMA, Echo Chemical Co., Ltd., Taiwan) was used to prepare the grafted functional polymer.
Sample preparation
Two blend compositions of TPU/OBC/PCL (50/30/20) and (70/20/10) with or without GMA and peroxide modifications were prepared. TPU and PCL were predried for 24 h at 50 °C in a vacuum oven prior to mixing. Blending processes were all conducted under 50 rpm. For the preparation of TPU/OBC/PCL blends, OBC/PCL blends were premixed in an internal mixer (Brabender, Brabender® 815,605, Duisburg, Germany) for 7 min at 135 °C, followed by mixing with TPU for another 4 min at 180 °C. The grafting reaction of OBC with 1.5 phr GMA containing 0.15 phr of peroxide was carried out for 7 min at 135 °C. The mixing of OBC-g-GMA and PCL was then performed afterwards, followed by mixing with TPU in a similar condition as described earlier to prepare TPU/OBC-g-GMA/PCL. A similar procedure was also conducted to prepare the corresponding blends of TPU/OBC/PCL-D and TPU/OBC-g-GMA/PCL-D blends containing 0.5 phr dicumyl peroxide (D representing the addition of peroxide). The samples were then hot-pressed in a compression molding machine (Feng Sheng, FS-1H1C., Taichung City, Taiwan) for 5 min at 180 °C. Tensile test specimens complying with the ISO-37 Type (III) standard were then prepared through a die cut. Before any measurements, the samples were stored in a vacuum drier at least one day.
Measurements
Structure and morphological characterizations
For the gel content measurement, at first, the continuous agitation of samples in boiling p-xylene was carried out to dissolve uncrosslinked PCL and OBC. Second, continuous agitation of samples in boiling THF was carried out to extract TPU. Then, the ratio of the weight of dried insoluble samples and the weight of samples before swelling was used to calculate the gel content. A scanning electron microscope (SEM, Tescan, 5136MM, Brno, Czech Republic) was performed on fractured surfaces of the samples sputtered with gold to evaluate the compatibility of the samples with GMA or peroxide modification.
Thermal characterizations
The glass transition temperature (Tg) was determined via a dynamic mechanical analyzer DMA (Perkin Elmer, Pyris Diamond, Waltham, USA) under a tension mode under a frequency of 1 Hz at a heating rate of 5 °C/min from −80 to 100 °C. The crystallization temperature (Tc) and melting temperature (Tm) were measured using a DSC (DuPont, TA Q10, Delaware, USA) at a cooling rate of 20 °C/min from 250 to −80 °C and a heating rate of 20 °C/min from −80 to 250 °C, respectively. The crystallinity was calculated by taking the heat of fusion divided by the enthalpy required for 100% crystallinity for PCL (134.9 J/g) and OBC (290 J/g), respectively [27, 28].
Shape-memory creation procedures (SMCP)
Shape memory measurements were conducted using an Instron 4469 (Boston, USA). For the dual-shape and triple-shape memory creation procedures, two programming processes were carried out, including program Ι and program II, respectively. Figure 1a illustrates the typical stress-strain-temperature curves for program I of TPU/OBC/PCL 50/30/20. This program consisted of only one deformation step. The original sample (shape C, εC) was heated to Td = 130 °C with the deformation strain of 80% (εAload), and then fixed by cooling to room temperature. Five min later, the load was removed to record the residual strain (εA) to attain the temporary shape A. On the recovery protocol, the sample was heated all the way to 130 °C, and allowed to recover from shape A (εA) to permanent shape C (εCrec). The shape fixing and recovery ratios were calculated as follows [29],
Figure 1b was the typical stress-strain-temperature curves illustration for program II of TPU/OBC/PCL 50/30/20. Two sequential steps of deformation were performed to attain two different temporary shapes. For the first step, the original sample (shape C) was loaded up to a strain of 60% (εBload) at the high deformation temperature of Td1 = 130 °C (140 or 150 °C), and then fixed by cooling to the low deformation temperature of Td2 = 65 °C. After 5 min, the load was removed to record (εB) to attain the temporary shape B. Secondly, the sample was loaded up to a strain of 80% (εAload) at 65 °C, and then fixed by cooling to room temperature. After 5 min, the load was removed to record (εA) to attain the temporary shape A. On the recovery protocol, the first recovery occurred from shape A (εA) to shape B (εBrec) by heating the sample to 65 °C. The second recovery from shape B (εBrec) to shape C (εCrec) occurred at 130 °C (140 or 150 °C). The shape fixing and recovery ratios were calculated as follows [30],
Results and discussion
The selection of the investigated blend ratios was based on their shape memory performances in terms of shape fixing ratio and shape recovery ratio in a dual shape memory process. As mentioned earlier, TPU was served as the fixed phase, and OBC and PCL were both served as switching phases, we have considered the complex ternary blend compositions involving TPU to blend with (OBC/PCL) components with the representative compositions of TPU/(OBC/PCL) blends at 100/(0), 70/(20/10), 50/(30/20), 30/(40/30), and 0/(60/40) corresponds to TPU/(OBC + PCL) blends at 100/(0), 70/(30), 50/(50), 30/(70), and 0/(100), respectively. In fact, there are numerous blend compositions for OBC/PCL blends, herein the amount of OBC close to ca. 60% in each OBC/PCL blend composition was chosen based on our previous work on the shape memory OBC/PCL blend composition at 60/40 under the deformation temperature of 65 °C for its best composition in consideration of the shape memory performance [31]. Thus, TPU blended with OBC/PCL component under this consideration was processed as described in the mixing sequence in the experimental section. Further, a preliminary study on the blend composition of TPU/(OBC/PCL) at the deformation temperature of 130 °C indicated that a rather low shape fixing ratio (65.2 ± 2.0%) for high content of TPU, such as pure TPU. This resulted from the lack of sufficient stabilizing force to withstand the high recovery force of TPU. As for samples for high content of (OBC/PCL), the TPU/(OBC/PCL) blend at 30/(40/30) had a rather low shape recovery ratio (59.3 ± 4.6%), even though with high shape fixing ratio (92.5 ± 0.9%). In addition, the TPU/(OBC/PCL) blend at 0/(60/40) was not capable of showing any shape memory behavior as the sample flowed at the temperature above the melting temperature of OBC and PCL during the test. Thus, only TPU/ (OBC/PCL) blends at 70/(20/10) and 50/(30/20) were chosen as typical compositions in this study in order to investigate the shape memory performance further. For simplicity, the parenthesis of (OBC/PCL) was omitted throughout the text. Another work on other detail compositions of OBC and PCL to couple with TPU is necessary in order to have a deeper understanding on their shape memory performance based on the current finding.
Gel content
The gel content of TPU/OBC/PCL blends with or without modifiers was listed in Table 1. Peroxide was known to initiate macroradicals for crosslinking, chain scission, and grafting reaction. Herein a small amount of peroxide (0.15 phr) was used to graft GMA on OBC. For brevity, the spectrum of OBC-g-GMA was omitted here. OBC-g-GMA didn’t show a measurable amount of gel content during the grafting process, indicating an efficient grafting process without much crosslinking. Thus, for the blends modified with GMA, the gel content was quite limited as well. For systems with the additional DCP (0.5 phr), peroxide tended to modify PCL to generate macromolecular radicals and crosslink OBC as well. Therefore, the gel content was slightly higher than that of those systems without 0.5 phr peroxide. Among them, for the systems modified with OBC-g-GMA and additional peroxide, the gel content was among the highest cases, which was attributed to the increased interaction of PCL and OBC through chain extension and possible OBC-co-PCL copolymer formation along with some amount of crosslinks. However, overall the gel content was still considerably small in comparison with normal gel content, more than 80%, for lightly crosslinking elastomers. Thus, the prepared blends are generally considered as green blends with easy of recycling in a sense. Note that the optimum compatibilizer concentrations were not considered at this moment, only a direct replacement of OBC with OBC-g-GMA and/or suitable amount of peroxide based on the previous own work and literature [31, 32] were employed here. Yet, a higher amount of peroxide was known to have an intendancy to form crosslinked blends further, which was against the current purpose to develop reprocessable SMP blends in this work. These first OBC-based multi-shape memory eco-blends are intended for the expansion of SMP blends, and another work is necessary for considering other materials, compositions, and process variables based on the current findings.
Morphology
The fractured surface of TPU/OBC/PCL blends with or without modification was shown in Figs. 2 and 3. For the 50/30/20 systems without any modifiers in Fig. 2a, the image showed the fractured surface of cocontinuous-like morphology, with loosely interconnecting big cavities and some dispersed spherical domains. The compatibility tended to increase with the replacement of the OBC-g-GMA modifier as seen in Fig. 2b, as evidence of more compact interconnecting structure with smaller cavities. It was known that the epoxy groups could react with both hydroxyl and carboxyl terminal groups of the polyesters, preferentially with the carboxyl groups. These coupling reactions resulted in epoxy ring-opening reactions and the derived formation of hydroxyl side groups [32, 33]. Thus, it was expected that the epoxy groups on OBC-g-GMA could react with the terminal groups of polyester like PCL. The possible specific interaction of OBC-g-GMA with TPU was plausible as well. Thus, the results indicated an improved interaction between OBC-g-GMA with other polar component in the modified ternary blends. In addition, the modification of peroxide was even more effective in improving the compatibility further, as seen in Fig. 2c image, showing the rough fractured surface with reduced spherical domain and cavity size. Peroxide tended to initiate free radicals on OBC and PCL to promote the formation of OBC-co-PCL copolymer formation and improve the interfacial interaction in the peroxide-modified blends. The previous gel content measurement showed only a limited gel content for all samples with the addition of peroxide. Thus, the additional peroxide to induce copolymer formation was the dominant effect, rather than the crosslinking effect. Further, in combination of GMA and peroxide modifications, the results showed that a synergistic effect in enhancing the compatibility further was found as seen in Fig. 2d image. The interfacial cavity and domain size on the fractured surfaces reduced dramatically, leading to the formation of uniformly fractured surface with more compact structure. For the 70/20/10 systems in Fig. 3, the figure images indicated that the blends without any modifiers showed some dispersed elongated particles and cavities. The particle and cavity sizes became smaller and uniform with the added OBC-g-GMA modifier. A similar conclusion on the further improved compatibility with considerably reduced spherical domain and cavity size on the fractured surface was also reached for GMA and peroxide-modified blends in these blends. Thus, both 50/30/20 and 70/20/10 systems showed a significant improvement in the compatibility with the addition of OBC-g-GMA and peroxide modifiers.
Thermal characterization
To examine the effect of the GMA and peroxide modification on the thermal behaviors of TPU/OBC/PCL blends, the results of the crystallization temperature (Tc), melting temperature (Tm), and crystallinity obtained from a differential scanning calorimetry are listed in Table 2 and Figs. 4 to 5. Fig. 4a shows that the crystallization temperature (Tc) of neat resins and TPU/OBC/PCL blends (50/30/20) with or without modification during the cooling scan. The crystallization temperature of OBC-g-GMA was slightly lower than that of OBC due to its less regular molecular structure to increase the free energy for good-packing from the view point of general crystallization behaviors. However, the crystallization temperatures of OBC in the blends increased slightly with GMA and peroxide modification in comparison with neat OBC, indicating the other components could serve the role of nucleating agent in various degrees, especially due to the enhanced compatibility between OBC and TPU/PCL as seen in the morphological observation. The crystallization temperatures of PCL also increased slightly with modification on the blends in most cases. On the other hand, the peroxide effect in the crosslinking OBC and degrading PCL made the variation in the crystallization behavior complex. So, in consideration of those factors, the difference in the increment of crystallization temperature of PCL and OBC was only evident in the case of TPU/OBC-g-GMA/PCL-D, but was considered to be marginal for the rest of compositions. For the melting temperatures in Fig. 4b, the difference was even smaller, indicating the variation in the crystal domains was not limited even after GMA and peroxide modification. Note that the crystallinity of both OBC and PCL in the blends with or without modification decreased in comparison with that of pure resin. It appeared that the existence of other components limited the growth of nucleating moiety. In addition, it was known that the crystallinity depended on the degree of crosslinking density, and a higher degree of crosslinking would inhibit the regular packing of molecular chains [17, 27]. Also, the compatibilization also led to the chain extension effect through the copolymer formation. Both effects tended to reduce the crystallinity of blends with respect to that of pure resins. For the comparison in the crystallinity of blends with or without modification, the values were not significantly altered, perhaps due to the limited gel content. Thus, the later discussion of mechanical properties could rule out the effect of variation in the crystalline effect. A similar finding was also observed on the TPU/OBC/PCL (70/20/10) blend systems with or without modifications.
Dynamical mechanical properties
To evaluate the viscoelastic behaviors for the modified TPU/OBC/PCL blends, a dynamic mechanical analyzer was employed. The results of dynamic storage modulus and tan δ varying with test temperatures for neat resins are shown in Fig. 6. As PCL exhibited a high degree of crystalline region, its storage modulus ranked the highest value with respect to other elastomeric resins (TPU, OBC, and OBC-g-GMA) in the room temperature range. The glass transition temperatures from the tan δ peak maxima were recorded as well. TPU rendered the highest glass transition temperature of −27.7 °C. The tan δ curves of PCL near melting were omitted as the measurement was not feasible while the test temperature was close to the melting temperature of PCL. The results of dynamic storage modulus and tan δ varying with test temperatures for modified blends at two different compositions are shown in Table 3 and Figs. 7 and 8. For the TPU/OBC/PCL (50/30/20) composition with or without modification, the insignificant variation in the storage modulus of TPU/OBC-g-GMA/PCL was observed even with the good dispersion of GMA-modified OBC component. Overall, storage modulus of TPU/OBC-g-MA/PCL-D modified blends gave the highest modulus in all cases. It was well known that peroxide could generate the radicals for crosslinking or chain scission of the molecular chains [34,35,36,37,38]. Thus, the decomposed peroxide could produce OBC and PCL macroradicals via the hydrogen abstraction from the main chain. At least three possible reactions could take place. First OBC or PCL macroradicals could crosslink with each other to form crosslinked OBC or PCL. Secondly, there was a tendency of PCL macroradicals to degrade via β chain scission normally found for the degradation process of polypropylene and polyesters, leading to degraded PCL. Thirdly, interchain coupling reactions from OBC and PCL macroradicals resulted in the formation of OBC-co-PCL copolymer. The interaction between these reactions was kind of complicated. This small increment in modulus was still considered within the experimental error in considering the complicated variation in the molecular structure. However, from the SEM observation, the compatibility was greatly improved with the addition of peroxide. Thus, the peroxide modifications were still quite helpful despite of the small degree of modulus increment. Besides the storage modulus evaluation, tan δ values were determined. The glass transition temperatures of OBC/PCL components were indiscernible and those values of blends were not clear to be determined. Although the respective glass transition temperature varied slightly due to the complicated ternary blends, the compatibility improvement via SEM observation was still visible. A similar conclusion was drawn for the TPU/OBC/PCL (70/20/10) systems with or without modifications.
Shape memory result
Most recent developments on the triple-shape memory crosslinked blends inevitably encountered the problem of recycling issue [17,18,19,20]. This work is the first environmental OBC-based triple-shape memory polymer eco-blends, especially in combination with biobased TPU and polycaprolactone (PCL). Two programming processes were implemented including one step programming (program I) to prepare dual-shape blends and the two-step programming (program II) to produce triple-shape blends.
Dual-shape programming
For the program I process, the dual-shape behavior was investigated first to have a better understanding on these ternary blends. The results are shown in Table 4. In this process, the switching temperature was set at 130 °C, instead of two switching temperatures as seen in the latter program II process. For TPU/OBC/PCL (50/30/20) blends with or without modifications, the samples were predeformed directly to a strain of 80% at 130 °C above the melting temperatures of OBC and PCL. The crystalline region of TPU acting as the physical crosslink points prevented the complete flow of the blends at this deformation temperature and stored the internal strain energy. This region was also partially dislocated and orientated like the deformed amorphous region through molecular orientation with low configurational entropy state. And when samples were cooled down to room temperature, the oriented amorphous and crystallized regions were fixed and stabilized to give the permanent set for producing a temporary shape. The shape fixing ratios (Rf) were pretty high up to 96.3% for the TPU/OBC-g-GMA/PCL-D blend. It seemed that the modification was helpful in improving the fixing condition of the TPU/OBC/PCL blend due to the increased compatibility of modified ternary blends. To investigate the recovery process, the samples were then heated again to regain their shapes at the recovery temperature of 130 °C. During the recovery process, the stored internal energy and configurational entropy loss were relieved upon heating above the melting temperatures of OBC and PCL. The highest Rr value reaching 91.2% was attained for the GMA and peroxide modified blend. Likewise, the TPU/OBC/PCL blends (70/20/10) with or without modifications were evaluated. Relatively lower Rf was found in comparison with previous blends with higher OBC and PCL content serving as the switching phase. The higher amount of TPU didn’t give higher recovery ratio, but instead slightly lower Rr. This might attribute to the morphology difference, as a cocontinuous-phase blend was reported to have better shape memory performance [39], even though the difference in the Rr was limited. The previous observation on the morphology of the blends partly revealed a cocontinuous-like phase for TPU/OBC/PCL blend (50/30/20), if one considered TPU as the fixed phase and OBC/PCL (30/20) as the switching phases. However, even though the modified blends didn’t reveal the clear contrast in the morphology due to the increased compatibility through the GMA and peroxide modification, this rational was still quite informative.
Triple-shape programming
For the two-step programming, PCL with low crystalline temperature and OBC with medium crystalline temperature were served as the switching phases, and TPU with high crystalline temperature was chosen as the fixed phase. The shape fixing ratios (Rf) and shape recovery ratios (Rr) of each step for two TPU/OBC/PCL blends with or without modifications are shown in Table 5. The original samples (C) were first deformed up to a strain of 60% at 130 °C, and then cooled down to 65 °C to fix their temporary shapes (B). This high temperature of 130 °C was set above the melting temperature of OBC (ca. 120 °C) and PCL (ca. 54 °C). During the deformation at high temperature, the TPU molecular chains with dislocated crystalline domains tended to deform easily with some degree of molecular orientation, and crystalline regions of OBC and PCL were all melted and integrated into amorphous regions, leading to the high degree of dislocation of crystalline domains and orientated molecular chains. When samples were cooled to the temperature below the melting temperature of OBC with relieved stress, high degree of permanent set with high shape fixing ratio was observed. Thus, TPU/OBC/PCL blends (50/30/20) with or without GMA or peroxide modifications gave high Rf(C→B) values up to 92.1%, as the OBC was capable of stabilizing its crystalline domain in the orientated state and PCL still remained in the flow state to dissipate the restoring force imposed by the TPU. To fix other temporary shapes (A), the samples were then loaded again to a strain of 80% at 65 °C, and cooled down to room temperature. At this stage, orientated PCL molecular chains could crystallize and fix in a temporary state as well, but the restoring force through the physical crosslinking effect to possess elastic behaviors was now not only from TPU crystalline phase, but also previously stabilized OBC crystalline domain as well. Therefore, slightly lower values Rf(B→A) than Rf(C→B) were observed for the blends, as PCL was the only phase to stabilize its crystalline domain to overcome the restoring effect from TPU and OBC.
To further investigate their recovery behaviors, the samples at room temperature were heated to 65 °C to regain their shapes, Rr(A→B) values were recorded at this stage. As this low recovery temperature was only above the melting temperature of PCL, the previously stored internal strain energy and low configurational state entropy for deformed PCL at low switching temperature was easily relieved upon heating to recover to their temporary shapes (B). However, surprisingly, the Rr(A→B) values were more than 100% in all prepared blends. This interesting feature was also observed for some triple-shape memory polymer systems [13]. It appeared that the first triggering (or recovery) process also induced the recovering process of the predeformed components at high deformation temperature, which led to the abnormally high recovery ratio. To complete the recovery process, the samples were heated to high recovery temperature above the melting temperature of OBC and PCL and Rr(B→C) values were recorded as well. These values were essentially lower than first recovery ratios due to the extensive recovery on the first recovery process. Additional stored internal strain energy and configurational state entropy loss contributed from predeformed OBC and TPU at the high deformation temperature were regained through this recovery process.
Overall, the effect of GMA and peroxide modification did show some variation on these shape fixing ratios and recovery ratios in comparison with neat TPU/OBC/PCL blends (50/30/20), however the difference on each modification process was quite limited for modified blends. The triple-shape or multi-shape could be tailored by varying the modification process in a different degree. To further investigate the variation of composition, TPU/OBC/PCL blends (70/20/10) with or without GMA and peroxide were conducted. In general, owing to the low OBC and PCL content, therefore the corresponding Rf(C→B) and Rf(B→A) values were lower than those of blends containing high OBC and PCL content, as the fixing condition was more closely related to disrupted crystalline domains of OBC and PCL besides the disentangled amorous regions. In addition, a similar conclusion was drawn for the interesting high Rr(A→B) values for all blends. However, these values were even relatively higher than those of TPU/OBC/PCL blends (50/30/20) with or without modification, as the first recovery condition appeared to associate with higher amount of predeformed components (TPU and OBC) at high deformation temperature in these blends containing low PCL content. Still, the detail mechanisms remained a challenge to those triple or multiple-shape memory polymers.
Effect of deformation temperatures
In order to further investigate the development of triple or multiple-shape behavior, the effect of the first deformation temperatures, such as 130, 140, or 150 °C, was considered for the representative (50/30/20) and (70/20/10) blends with GMA and peroxide modification in Fig. 9, as this blend gave the highest shape recovery ratio more than 90% in the course of dual-shape development, which were discussed in the program I process. The low deformation temperature of 65 °C remained the same. The effect of increased temperature difference between high and low deformation temperatures was shown in Fig. 9a for two different compositions of blends. The shape fixing ratios in two fixing stages, Rf(C→B) and Rf(B→A), for the modified TPU/OBC/PCL (50/30/20) blend didn’t vary much with the effect of temperature difference. However, a measurable increment on Rf(B→A) was found for modified TPU/OBC/PCL (70/20/10) blend. It was reported in our recent work [40] regarding the dual-shape PLA/TPU blends that at an optimum deformation temperature the polymer chains with orientated crystalline domains tended to move more easily with high degree of molecular orientation. Thus, higher amount of fixed crystalline phase formed at high temperature would help to induce the higher molecular orientation degree in the second fixing stage. Likewise, Li et al. [41] also reported this phenomenon in their triple-shape system, which indicated a higher amount of memory portions was “fixed” upon cooling for higher deformation temperature, yet the number of “non-fixed” memory portions remained unchanged since the low deformation temperature was still the same. By increasing the high deformation temperature, the fixing degree at different stages could be adjusted. In our case, as the lower amount of PCL in the modified TPU/OBC/PCL (70/20/10) blend was used, so this increased deformation temperature effect to enhance the fixing ratio in the second fixing stage could compromise the lower fixing ratio due to lower PCL composition compared with that of modified TPU/OBC/PCL (50/30/20) blend.
For the effect of increasing deformation temperature on the recovery ratio of modified blends, the results are shown in Fig. 9b. Regarding the modified TPU/OBC/PCL (50/30/20) blend, the first recovery ratio, Rr(A-B) decreased slightly with increasing deformation temperature. However, the recovery ratio in this stage was still more than 100%. A similar situation was also found for the modified TPU/OBC/PCL (70/20/10) blend, but to a more significant decrement. As previously discussed, a slightly higher fixing degree in the second fixing stage for the blend containing low PCL content due to the increased molecular orientation effect induced at higher deformation temperature on the first stage, thus the recovery efficient was less prominent at low recovery temperature. Interestingly, both modified blends showed the limited influence from the increased deformation temperature on the recovery ratio in the second stage, Rr(B-C). Apparently, the molecular orientation induced at high deformation temperature was still limited in terms of small entropy configuration variation at high temperature. Note that the thermal history in the shape memory was important to the shape memory performance as well, thus all samples in this study were air-cooled directly from the compression molding under the same thermal history. In addition, similar air-cooling conditions were used during the shape memory tests. Our previous work [42] about the annealing effect on the shape memory performance of the TPU/polylactic acid blend is included to demonstrate the significance of thermal history. It is worth of investigating this thermal history effect in the other work.
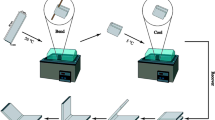
Figure 10 shows the illustration of dual-shape for a typical blend TPU/OBC-g-GMA/PCL-D blend (50/30/20) under shape program I. The temporary sample shape was first shaped at 130 °C followed by cooling to room temperature at 130 °C, and then recovered to the permanent shape at 130 °C. In a very short time of a few seconds, the predeformed sample was recovered to the original shape. Fig. 11 shows the illustration of various shapes for a typical blend TPU/OBC-g-GMA/PCL-D blend (50/30/20) under shape program II. The permanent shape almost completely recovered, even though no fully crosslinked systems were involved, as reported recently by Gu et al. on their TPU/polylactide–polytetramethylene ether (PLA-PTMEG) blend system [26]. In this work, with suitable adjustment on the composition and shape memory programming of TPU/OBC/PCL blends (70/20/10 and 50/30/20), the multi-shape memory effect could be tailored. Most of all, through this subtle adjustment on the temperature difference between high and low deformation temperatures, the theoretical multi-shape memory shape could be readily tailored to meet different applications.
Conclusions
Thermoplastic polyurethane (TPU)/olefin block copolymer (OBC)/polycaprolactone (PCL) blends (70/20/10 and 50/30/20) were melt-blended to form the first environmental OBC-based triple-shape memory polymer blends. Two compatibilizers, OBC-g-glycidyl methacrylate (OBC-g-GMA) and dicumyl peroxide, effectively showed a synergistic effect in enhancing the compatibility. The thermal analysis indicated that crystallinity of both OBC and PCL in the blends with or without modification decreased in comparison with that of pure resin due to the existence of other components and compatibilization to limit the growth of nucleating moiety. Two types of shape memory behaviors could be adjusted, including dual-shape and triple shape blends. For dual-shape behaviors, the shape fixing ratios (Rf) and shape recovery ratio (Rr) were up to 96.3% and 91.2% for the GMA and peroxide-modified blends (50/30/20). For triple-shape behaviors, both TPU/OBC/PCL blend compositions with or without GMA/peroxide modifications gave high Rf(C→B) values in the first fixing stage, but slightly lower values Rf(B→A) in the second fixing stage, especially for (70/20/10) case. On the other hand, a reverse trend was observed for two recovery stages. Through a subtle adjustment on the temperature difference between high and low deformation temperatures, the triple-shape or multi-shape could also be tailored besides by varying the modification process in a different degree.
References
Ratna D, Karger-Kocsis J (2008) Recent advances in shape memory polymers and composites: a review. J Mater Sci 43(1):254–269
Leng J, Lan X, Liu Y, Du S (2011) Shape-memory polymers and their composites: stimulus methods and applications. Prog Mater Sci 56(7):1077–1135
Meng H, Li G (2013) A review of stimuli-responsive shape memory polymer composites. Polymer 54(9):2199–2221
Lewis CL, Dell EM (2016) A review of shape memory polymers bearing reversible binding groups, J. Polym. Sci., part B: Polym. Phys 54(14):1340–1364
Wang H, Taha A, Chum SP, Hiltner A, Baer E (2007) Comparison of block and random ethylene-octene copolymers based on the structure and elastomeric properties. Annu Tech Conf Conf Proc 2:1181–1185
Zhang Q, Feng J (2013) Difunctional olefin block copolymer/paraffin form-stable phase change materials with simultaneous shape memory property. Sol Energy Mater Sol Cells 117:259–266
Zhang Q, Cui K, Feng J, Fan J, Li L, Wu L, Huang Q (2015) Investigation on the recovery performance of olefin block copolymer/hexadecane form stable phase change materials with shape memory properties. Sol Energy Mater Sol Cells 132:632–639
Zhang Q, Hua W, Feng J (2016) A facile strategy to fabricate multishape memory polymers with controllable mechanical properties. Macromol Rapid Commun 37(15):1262–1267
Pretsch T (2010) Durability of a polymer with triple-shape properties. Polym Degrad Stab 95(12):2515–2524
Luo X, Mather PT (2010) Triple-shape polymeric composites (TSPCs). Adv Funct Mater 20(16):2649–2656
Zotzmann J, Behl M, Feng Y, Lendlein A (2010) Copolymer networks based on poly(ω-pentadecalactone) and poly(ϵ-caprolactone)segments as a versatile triple-shape polymer system. Adv Funct Mater 20(20):3583–3594
Ware T, Hearon K, Lonnecker A, Wooley KL, Maitland DJ, Voit W (2012) Triple-shape memory polymers based on self-complementary hydrogen bonding. Macromolecules 45(2):1062–1069
Narendra Kumar U, Kratz K, Behl M, Lendlein A (2012) Shape-memory properties of magnetically active triple-shape nanocomposites based on a grafted polymer network with two crystallizable switching segments. Express Polym Lett 6(1):26–40
Ge Q, Luo X, Iversen CB, Mather PB, Dunn ML, Qi HJ (2013) Mechanisms of triple-shape polymeric composites due to dual thermal transitions. Soft Matter 9(7):2212–2223
Bai Y, Zhang X, Wang Q, Wang T (2014) A tough shape memory polymer with triple-shape memory and two-way shape memory properties. J Mater Chem A 2(13):4771–4778
Wu Y, Hu J, Zhang C, Han J, Wang Y, Kumar B (2015) A facile approach to fabricate a UV/heat dual-responsive triple shape memory polymer. J Mater Chem A 3(1):97–100
Cuevas JM, Rubio R, Germán L, Laza JM, Vilas JL, Rodriguez M, León LM (2012) Triple-shape memory effect of covalently crosslinked polyalkenamer based semicrystalline polymer blends. Soft Matter 8(18):4928–4935
Radusch HJ, Kolesov I, Gohs U, Heinrich G (2012) Multiple shape-memory behavior of polyethylene/polycyclooctene blends cross-linked by electron irradiation. Macromol Mater Eng 297(12):1225–1234
Zhao J, Chen M, Wang X, Zhao X, Wang Z, Dang ZM, Ma L, Hu GH, Chen F (2013) Triple shape memory effects of cross-linked polyethylene/polypropylene blends with cocontinuous architecture. ACS Appl Mater Interfaces 5(12):5550–5556
Hoeher R, Raidt T, Krumm C, Meuris M, Katzenberg F, Tiller JC (2013) Tunable multiple-shape memory polyethylene blends. Macromol Chem Phys 214(23):2725–2732
Wang Z, Zhao J, Chen M, Yang M, Tang L, Dang ZM, Chen F, Huang M, Dong X (2014) Dually actuated triple shape memory polymers of cross-linked polycyclooctene-carbon nanotube/polyethylene nanocomposites. ACS Appl Mater Interfaces 6(22):20051–20059
Samuel C, Barrau S, Lefebvre JM, Raquez JM, Dubois P (2014) Designing multiple-shape memory polymers with miscible polymer blends: evidence and origins of a triple-shape memory effect for miscible PLLA/PMMA blends. Macromolecules 47(19):6791–6803
Kolesov I, Dolynchuk O, Radusch HJ (2015) Shape-memory behavior of cross-linked semi-crystalline polymers and their blends. Express Polym Lett 9(3):255–276
Karger-Kocsis J, Kéki S (2014) Biodegradable polyester-based shape memory polymers: concepts of (supra)molecular architecturing. Express Polym Lett 8(6):397–412
Kashif M, Chang YW (2015) Triple-shape memory effects of modified semicrystalline ethylene–propylene–diene rubber/poly(ɛ-caprolactone) blends. Eur Polym J 70:306–316
Gu S-Y, Liu L-L, Gao X-F (2015) Triple-shape memory properties of polyurethane/polylactide–polytetramethylene ether blends. Polym Int 64(9):1155–1162
Pandini S, Baldi F, Paderni K, Messori M, Toselli M, Pilati F, Gianoncelli A, Brisotto M, Bontempi E, Riccò T (2013) One-way and two-way shape memory behaviour of semi-crystalline networks based on sol–gel cross-linked poly(ε-caprolactone). Polymer 54(16):4253–4265
Wang HP, Chum SP, Hiltner A, Baer E (2009) Deformation of elastomeric polyolefin spherulites. J Polym Sci Part B Polym Phys 47(13):1313–1330
Bellin I, Kelch S, Langer R, Lendlein A (2006) Polymeric triple-shape materials. PNAS 103:18043–18047
Heuchel M, Sauter T, Kratz K, Lendlein A (2013) Thermally induced shape-memory effects in polymers: quantification and related modeling approaches. J Polym Sci Part B Polym Phys 51(8):621–637
Lai S-M, Wang X-F (2017) Shape memory properties of olefin block copolymer (OBC)/polycaprolactone (PCL) blends. (Early view). J Appl Polym Sci 134:45475
Pai F-C, Lai S-M, Chu HH (2013) Characterization and properties of reactive poly(lactic acid)/polyamide 610 biomass blends. J Appl Polym Sci 130(4):2563–2571
Al-Itry R, Lamnawar K, Maazouz A (2012) Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym Degrad Stab 97(10):1898–1914
Mishra JK, Chang YW, Kim DK (2007) Green thermoplastic elastomer based on polycaprolactone/epoxidized natural rubber blend as a heat shrinkable material. Mater Lett 61(17):3551–3554
Semba T, Kitagawa K, Ishiaku US, Kotaki M, Hamada HJ (2007) Effect of compounding procedure on mechanical properties and dispersed phase morphology of poly(lactic acid)/polycaprolactone blends containing peroxide. J Appl Polym Sci 103(2):1066–1074
Ma P, Hristova-Bogaerds DG, Lemstra PJ, Zhang Y, Wang S (2012) Toughening of PHBV/PBS and PHB/PBS blends via in situ compatibilization using dicumyl peroxide as a free-radical grafting initiator. Macromol Mater Eng 297(5):402–410
Ji D, Liu Z, Lan X, Wu F, Xie B, Yang M (2014) Morphology, rheology, crystallization behavior, and mechanical properties of poly(lactic acid)/poly(butylene succinate)/dicumyl peroxide reactive blends. J Appl Polym Sci 131(3):39580–39587
Han C, Ran X, Su X, Zhang K, Liu N, Dong L (2007) Effect of peroxide crosslinking on thermal and mechanical properties of poly(ε-caprolactone). Polym Int 56(5):593–600
Zhang H, Wang H, Zhong W, Du Q (2009) A novel type of shape memory polymer blend and the shape memory mechanism. Polymer 50(6):1596–1601
Lai S-M, Lan Y-C (2013) Shape memory properties of melt-blended polylactic acid (PLA)/thermoplastic polyurethane (TPU) bio-based blends. J Polym Res 20:140–147
Li J, Xie T (2011) Significant impact of thermo-mechanical conditions on polymer triple-shape memory effect. Macromolecules 44(1):175–180
Lai SM, Wu WL, Wang YJ (2016) Annealing effect on the shape memory properties of polylactic acid (PLA)/thermoplastic polyurethane (TPU) bio-based blends. J Polym Res 23(5):1–13
Acknowledgments
The authors are grateful to Mr. Yen-Ju Chen and Geng-Lun Guo for helping manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, SM., You, PY., Chiu, Y.T. et al. Triple-shape memory properties of thermoplastic polyurethane/olefin block copolymer/polycaprolactone blends. J Polym Res 24, 161 (2017). https://doi.org/10.1007/s10965-017-1319-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1319-z