Abstract
A flame retardants containing phosphorus-silicon, DOPO-VTS, was synthesized and incorporated into polymethyl methacrylate (PMMA) matrix through sol-gel process at different loadings. The results from Fourier Transform Infrared Spectroscopy (FTIR), Scanning Electron Microscope (SEM) and Differential Scanning Calorimetry (DSC) showed that silicon particle were formed and dispersed well in the PMMA matrix. The addition of DOPO-VTS can not only enhance the flame retardancy of PMMA but also improve the thermal stability of PMMA. When compared to PMMA, the addition of only 15wt% DOPO-VTS results in 28.5% decrease in pHRR. Moreover, 15.0 wt% DOPO-VTS results in 32.0 °C increase in half degradation temperatures (T0.5). The results of Hot Stage Microscopy (HSM) and FTIR showed that phosphorus-containing compound and the silicon crystal were formed in the char layers during the pyrolysis process, and the char layers can effectively prevent the degradation of PMMA/silicon particle composites. It's believed that this research will stimulate further efforts in silicon particle as the based flame retardants in different polymers for the property reinforcements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past few years much attention has been focused on improving the properties of polymer by using nano-fillers. Only a small amount of nano-fillers imparted can bring the polymers with excellent properties, mechanical performance, gas barrier, thermal stability and flame retardant properties. [1–3] However, the nano-fillers tend to re-aggregate when they are introduced into polymers. In order to improve the dispersion of nano-fillers in polymer matrix, many methods, such as in situ polymerization, solution intercalation or melt intercalation, have been developed. [4–6] Among these methods, in situ polymerization is considered as a very effective method to prepare polymer nanocomposites with good dispersion of the nano-fillers. As far as we are concerned, it’s reported that many nano-fillers, such as carbon nanotubes (CNTS), silicon nanoparticle, expanded graphite (EG), montmorillonite, layered double hydroxides (LDH) or zirconium phosphate, have been applied in the flame retardancy of polymer materials. Among those nano-fillers, the silicon based organic/inorganic nano-fillers are promising because it contains organic groups, which results in the homodispersion of the nano-fillers in the polymer matrix.
The silicon based organic/inorganic nano-fillers can be synthesized by the sol-gel method. The silicon atoms usually contains the organic functional groups, which can provide the reactive groups for improving the interface interaction between the nano-fillers and polymer matrix. Through sol-gel method, the organic molecules were introduced inside silicon networks, which can solve the compatibility problems that the polymer nanocomposites are usually faced with. [7] Compared with the conventional methods, the organic/inorganic silicon based particle prepared through sol-gel methods exhibit four advantages: (1) the cross-linked networks are formed during the sol-gel process; (2) strong chemical interactions exist between inorganic phases and organic phases; (3) the monodispersion of silicon particle can be achieved; (4) the organic/inorganic silicon particle can be obtained under mild characteristics. Based on the four advantages, the as-prepared polymer nanocomposites present improved thermal stability and mechanical properties, but as those for flame-retardant properties, the effects are not obvious. Generally, introducing flame retardant elements into the silicon hybrids is a promising way to improve the flame retardant properties of the polymer materials.
Among various flame retardants elements, the silicon-based flame retardants are important flame retardant elements in the process of flame retardant polymers. When heated, the silicon elements will migrate onto the surface of char layers due to their low surface energy. [8, 9] The silica-carbon char layers with high thermal stability are formed to prevent the residues from further thermal decomposition. Moreover, many researchers have made efforts to develop phosphorus based flame retardants with high flame-retardant efficiency and thermal stability. [10–12] It’s reported that phosphorus-functionalized polymers, whether in the phosphine oxide form, in the phosphonate or in the phosphinate form, can improve the flame retardant properties and atomic oxygen resistance of polymer. The char formed on the surface of materials will act as a protective barrier, which can prevent the gaseous products from escaping into the air and preventing the polymer matrix from heat. Among those phosphorus-containing flame retardants, 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) has drawn considerable attention because it has relatively high thermal stability and flame retarding efficiency. [13, 14] The phosphorus or silicon based flame retardants can be used individually as flame retardants (FRs). However, individually using flame-retardants endows polymer matrix with limited flame-retardant properties. Generally, the flame retardants combining phosphorus and silicon elements are promising in the flame retardancy of polymer materials. The flame retardants containing both phosphorus and silicon present good potential in improving the flame retardant efficiency.
PMMA, which has been widely used in building construction, optical lenses and optical cable, is an important thermoplastic polymer. It has excellent performances including high strength, good flexibility, dimension stability, weather resistance and so on, but it is always highly flammable and has very poor heat resistance. [15, 16] Those drawbacks limit the further use of PMMA in many desirable applications. In order to improve the flame retardant properties of PMMA, organo-phosphorus compounds are usually incorporated into the polymer matrix through melt blending or solution blending. However, those methods often lead to low flame retardant efficiency due to the poor distribution of the flame retardants in the polymer matrix. Thus, the chemical incorporation of the flame retardants through copolymerization or chemical modification is a viable approach to overcome those problems. As a result, developing novel flame retardants, which can simultaneously improve the thermal and flame retardancy properties of PMMA, has developed a good way to improve the fire safety of PMMA.
Inspired by the previous work, vinyl trimethoxy silane grafted by DOPO-VTS was synthesized and then incorporated into PMMA matrix at different loadings. The reinforcing effects of DOPO-VTS on the fire safety of PMMA were compared and investigated. The silicon base flame retardant is considered as a practical method for the production of high performance PMMA composites. It’s believed that this research will stimulate further efforts in silicon particle as the based flame retardants in different polymers matrix for the properties reinforcement.
Experimental
Materials
9,10-Dihydro-9-oxa-10-phosphaphenanthrene 10-oxide was obtained from Shandong Mingshan Fine Chemical Industry Co. Ltd. (Shandong, China). Vinyl trimethoxy silane was provided by Shenmao New Material Co. (Guangzhou, China) and was distilled before use. And Hydrochloric acid (HCl) was purchased from Shanghai Chemical Reagents Company of China, while tetrahydrofuran (THF), 2-Azobisisobutyronitrile (AIBN), methyl methacrylate (MMA) and 3-methacryloxypropyltrimethoxysilane were provided by Sinopharm Chemical Reagent Co., Ltd. MMA was used after reduced pressure distillation. All the other chemical reagent were used without purification.
Preparation of PMMA and PMMA/silicon particle composites
DOPO-VTS are synthesized according to our previous work. [17] PMMA and PMMA/silicon particle composites was prepared by free radical bulk copolymerization. Generally, 3-methacryloxypropyltrimethoxysilane (5.0 g) and MMA monomers (95.0 g) were added into a beaker with the assistance of sonication for 1 h. Once the mixtures became completely transparent, AIBN (0.2 g) was added to the mixtures, then the mixtures were stirred at 80 °C for about 1 h until a viscous paste was formed. Then, the viscous paste was then transferred into a mold and held at 60 °C for 48 h to complete the polymerization process and remove the unreacted MMA. The schematic process of the preparation process is presented in Scheme 1.
Preparation of flame retardant PMMA composites
The flame retardant PMMA composites were prepared through the sol-gel process. Typically, silicon modified PMMA copolymers and DOPO-VTS were added into a THF solution; the total weight of PMMA copolymers and DOPO-VTS was kept at 20 g; 5.0 ml H2O and 1.5 ml HCl were added into the mixtures as the catalyst and the mixtures were stirred mechanically for 6 h. During the sol-gel process, the inorganic silicon spheres were formed in situ by the hydrolysis and the condensation of DOPO-VTS. Then, the products were casted into aluminum dishes to gel at room temperature for another 24 h and the products are dried in vacuum drier at 60 °C for 20 h. The details of flame retardant PMMA composites with different content of DOPO-VTS are listed in Table 1.
Characterization
Nicolet 6700 FT-IR spectrophotometer was employed to characterize the composites and the char residue of the composites using thin KBr disc. The wavelength range was at the range of 4000 to 500 cm−1.
A SPEX-1403 laser Raman spectrometer through a argon laser line (514.5 nm) in a back-scattering geometry was used to character Raman spectra.
The morphologies of polymer nanocomposites were investigated by scanning electron microscopy (SEM, AMRAY1000B). The samples were sputter coated with a gold layer.
The thermo gravimetric analysis (TGA) was conducted to investigate the thermal stability of the composites, the instrument is a Q5000 thermo-analyzer instrument (TA Instruments Inc., USA). The samples are heated from room temperature to 500 °C at a linear heating rate of 20 °C/min in air.
The microscale combustion colorimeter (MCC, GOVMARK MCC-2) was adopted to study the combustion of the composites. Only about 5 mg of the composite were heated to 900 °C at the heating rate of 1 K/s.
Hot Stage Microscopy (HSM) was used to investigate the morphology of the composites during the thermal degradation process. The samples were heated from room temperature to 580 °C at the heating rate of 60 °C/min.
The crystallization behaviors of the composite were investigated by differential scanning calorimetry (DSC). The temperature of the composites were heated from 25 to 200 °C, then the temperature was decreased from 200 to 0 °C at 10 °C min−1. The data for crystallization behavior of the composites were obtained from the first heating and the second cooling, respectively.
Results and discussion
The structures of the PMMA and flame-retardant PMMA composites
Figure 1 shows the FTIR spectra of PMMA and flame-retardant PMMA composites. The characteristic bands of PMMA, such as 1242 and 1149 cm−1 for C-O, 1730 cm−1 for C = O, and 2949 cm−1 for C-H, appeared. However, when DOPO-VTS were incorporated into the PMMA matrix, some new absorption peaks appeared. The peak at 990 cm−1 for the P-O-Ph structure and the peak at around 1100 cm−1 for the Si-O-Si bond may be coincidence with PMMA matrix. [18] To investigate state of DOPO-VTS in PMMA matrix, the fractured sections of PMMA-3 at low temperature fractures were investigated by SEM, as shown in Fig. 2. As for the PMMA, the fractures surface exhibited an orderly fish striate structures, suggesting brittle failure of homogeneous materials. It’s obvious that the silica particle are formed in the PMMA matrix during the sol-gel process. Under higher magnification, it can be found that the silicon particle have good interfacial interaction with PMMA matrix. Moreover, the silicon particle are distributed uniformly on the fracture surface, which is due to the chemical bonds between the silicon particle and PMMA matrix. Generally, the uniform dispersion of the silicon particle in the PMMA matrix is largely attributed to the reactive sites between the PMMA matrix and the silicon particle. The organic groups on the surface of silicon particle could form strong interfacial interaction with the PMMA matrix, resulting in the good dispersion of the particle in the PMMA matrix.
TGA is used as a complementary technique for revealing the thermal behaviors of the sol-gel products of DOPO-VTS (FRs). The TGA of FRs in both air and nitrogen atmospheres are shown in Fig. 3. It is found that FRs is not thermally stable and subject to a mass loss in both nitrogen and air atmospheres at the initial stage, that’s because the polyhydroxy groups in the organic/inorganic FRs decompose easily at the initial temperature. As for the degradation in air atmosphere, the FRs started to degrade at 150 °C and decomposed completely at 600 °C. Similar to the decomposition behavior in air atmosphere, the organic/inorganic FRs is subject to mass loss at low temperature in nitrogen atmosphere, but the char residues of FRs in air atmosphere are higher than those in nitrogen atmosphere after 500 °C. Thus, due to the high char residues, FRs has the potential to improve the flame retardant properties of PMMA.
Thermal properties of PMMA and PMMA/silicon particle composites
The Tg of the polymer materials provided an important information to evaluate the segmental mobility of polymer chains. In order to verify the existence of silicon particle and investigate the influence of silicon particle on the segmental motions in hybrid materials, Tg of PMMA and PMMA/silicon particle composites were tested by DSC. The DSC curves of PMMA and PMMA/silicon particle composites are presented in Fig. 4 and the corresponding data are listed in Table 2. It is obvious that the Tg of PMMA/silicon particle composites shifted to higher temperature when DOPO-VTS was incorporated into PMMA matrix. The Tg value increases gradually from 98 °C to 110 °C when the DOPO-VTS content is increased to 15 wt%. Besides, the rest of the mixed PMMA systems all exhibit single Tg. The single Tg phenomenon indicates a homogeneous morphology of the PMMA system. The increase of Tg is due to the great restriction of PMMA chain motions, indicating good interfacial interaction between the silicon particle and PMMA chains. Moreover, the silicon based networks may play an important role in the restraining PMMA chain motions.
TGA is a technique for revealing the thermal stability and thermal degradation process of various polymer materials. The TGA curves of PMMA and PMMA/silicon particle in nitrogen atmosphere are shown in Figs. 5 and 6. The temperatures at which 10% (T0.1) weight loss occurs are used as the measure of the onset temperatures while the temperatures at which 50% (T0.5) weight loss occurs are regarded as the measure of half degradation temperatures. As for PMMA, the T0.1 of PMMA/silicon particle composites are lower than those of PMMA. That’s because the hydroxy in the silicon network is not thermal stable and degrades at lower temperature. Moreover, when DOPO-VTS is incorporated into the PMMA matrix, the main weight-loss moves to higher temperature. Furthermore, it can be observed that PMMA has almost no residues at 500 °C, while the corresponding char residues of PMMA composites at 500 °C rises from 3.2 wt% for PMMA-1 to 11.3 wt% for PMMA-3. Moreover, the half degradation temperatures (T0.5) of all PMMA/silicon particle composites are larger than those of PMMA. Only 5.0 wt% of DOPO-VTS added into the composites leads to 22 °C increase of T0.5. As for higher level of DOPO-VTS (15.0 wt%) in the PMMA matrix, the T0.5 of the composites are increased by 32.0 °C. Those results indicate that the enhanced endurance of PMMA copolymers against thermal oxidation at high temperature because is due to the charring of PMMA copolymers, which is a favorable factor for the flame retardancy of PMMA. [19, 20] Those indicate that the incorporation of DOPO-VTS into PMMA matrix can significantly enhance the thermal stability of the PMMA composites. As for PMMA, there is almost no residues when the temperature is higher than 500 °C in nitrogen atmosphere, whereas the corresponding residue amounts of PMMA-1, PMMA-2 and PMMA-3 are about 3.2 wt.%, 5.9 wt.% and 11.4 wt.%, respectively. That’s because the degradation of silicon-phosphorus containing groups degrade and form heat-resistant residues, which will result in high char residues. As the temperature increases, the char residues of PMMA composites do not degrade anymore, indicating that thermal stable char layers are formed at the temperature range of 500 °C ~ 700 °C. Those results indicate that the char residues can resist the temperature as high as 700 °C. The thermal stable char residues will limit the production of combustible gases and the diffusion of heat, resulting in the improved thermal stability of PMMA composites.
As for the TGA results of PMMA and PMMA/silicon particle composites in air atmosphere, it’s found that the degradation process of the PMMA composites are different from those in nitrogen atmosphere, as shown in Fig. 6. For example, the char residues of PMMA in air atmosphere at 500 °C is about 0.1 wt.%, and as those for the char residues of PMMA-3 in air atmosphere at 500 °C, the level is 13.3 wt.%. As for the T0.5 of the composites, the T0.5 of PMMA/silicon particle composites increased significantly. For example, the T0.5 of the PMMA is only 296 °C, after modified by DOPO-VTS, the T0.5 of the PMMA is increased to 371 °C, which is a 75 °C increment. As those for the PMMA-3 in nitrogen atmosphere, the increment is only 28 °C. Those indicate that the silicon particles have better effect in preventing the PMMA from degradation in air atmosphere than those in nitrogen atmosphere. Generally, the silicon networks and the good interfacial interaction between silicon particle and the PMMA matrix are hypothesized to be the main reasons for the improved thermal stability. The networks and the good interfacial interaction make it difficult for the segmental motion of polymer, when compared to PMMA.
Flame retardant properties of PMMA and PMMA/silicon particle composites
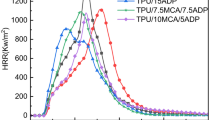
To further investigate fire safety of PMMA and PMMA/silicon particle composites, Micro Combustion Calorimeter (MCC), as a laboratory scale test to measure chemical properties related to fire through thermal analysis methods, is adopted. MCC can supply the key factors such as peak of heat release rate (pHRR) and total heat release (THR). The curves of HRR vs. tempearatures for PMMA and the composites are shown in Fig. 7. In the previous work, it’s reported that PMMA degraded mainly with the form of depolymerization and released highly flammable MMA monomers. [21, 22] Thus, this will result in a high value of pHRR (284.1 W/g) during the MCC test. From Fig. 7, the pHRR of PMMA composites shifted to lower values when the DOPO-VTS are incorporated into the PMMA matrix. Compared to PMMA, introduction of silicon particle in the PMMA matrix results in a 34% maximum decrease in pHRR. Thus, it can be concluded that the presence of silicon particle is responsible for the reduction of pHRR. On one hand, the synergist effects between phosphorus and silicon may be one key factor for the improvement of fire safety. On the other hand, the phosphorus in the PMMA composites can promote the formation of char layers, as suggested by the TGA results. The char layers will protect the underlying PMMA matrix from further degradation, resulting in reduced pHRR. However, as for the THR of the composites, the effect is not obvious. The PMMA do not release heat at 500 °C, but as those for PMMA/silicon particle composites, there are still heat release even at 550 °C, which indicate that the DOPO-VTS can reduce the heat release rate of combustion gases due to the formation of char layers, but the char layers will decompose at high temperature and combustible gases will be released.
Thermal oxidative degradation of PMMA/silicon particle composites
The changes of the microstructure in the condensed phase during the thermal oxidative degradation of the PMMA/silicon particle composites were investigated by a Hot Stage Microscopy (HSM). PMMA-3 are heated from room temperature to 580 °C at the heating rate of 60 °C/min, and during the thermal degradation process of PMMA/silicon particle composites, the microstructure changes are observed, as shown in Fig. 8. For the composites at 20 °C, it’s found that the surface is smooth. As the temperature increased, some uplifts appears on the surface of the composites, and when the temperature increased to 500 °C, the uplifts are full of the composites surface, suggesting that the composites degrade and the condensed char layers were formed. However, when the temperature are further increased to 580 °C, some lightspots appeared. It seems that the char layers are broken at high temperature. When the polarization was changed, crystals were found to have formed in the “lightspots” seen under the other light polarization under the microscope. In order to investigate the mechanism, the char residues of PMMA-3 after HSM test are investigated by FTIR, as shown in Fig. 9. The peaks at 3431 cm−1 are due to the hydroxyl in the char layers. The hydroxyl may be correspond to the hydroxyl of phosphoric acid or polyphosphoric acid, which are the thermal degradation products of the organic/inorganic silicon particles. Meanwhile, the sharp absorption peak appearing at 1644 cm−1 is assigned to the stretching vibrations of C = C in the aromatic compounds. Generally,the PMMA usually depolymerizes during the thermal degradation process and it hard for the PMMA to form the benzene rings. Thus, the aromatic compounds may originate from the benzene ring of the organic/inorganic silicon particles. What’s more, the peaks at 1100 cm−1 is wide, that’s because the characteristic peaks of Si-O-Si and P-O-Ph are coincidence with each other bonds. [23, 24] Generally, the FTIR and HSM results confirm that the silicon crystal is formed during the pyrolysis process. The phosphorus compounds and silicon crystal in the char layers will effectively inhibit the thermal degradation of PMMA/silicon hybrids composites.
Conclusion
In the present work, a series of PMMA/silicon particle composites with different silicon particle contents have been prepared successfully prepared through sol-gel process. The silicon particle are form during the sol-gel process and dispersed well in the PMMA matrix. The incorporation of silicon particle into the PMMA matrix results in great improvement in the thermal and flame retardant properties of PMMA. The homodispersion of silicon particle in the PMMA matrix and the good interfacial interaction between the silicon particle and the PMMA matrix are the two main factors for the improved fire safety. The phosphorus compound combining with the silicon crystal form a solid char layers and the solid char layers can effectively prevent the thermal degradation of PMMA/silicon hybrids composites. It’s believed that this research will stimulate further efforts in silicon particle as the based flame retardants in different polymers matrix for the properties reinforcement.
References
Podsiadlo P, Kaushik AK, Arruda EM, Waas AM (2007) Science 318:80–83
Du F, Scogna RC, Zhou W, Brand S, Fischer JE (2004) Macromolecules 24:9048
Tjong SC (2006) Mat Sci Eng R. Rep Vol 53:73
Alexandre M, Dubois P (2000) Mater Sci Eng R 28:1–63
Ray SS, Bousmina M (2005) Prog Mater Sci 50:962–1079
Kim H, Abdala AA, Macosko CW (2010) Macromolecules 43:6515
Badshah S, Airoldi C (2013) Thermochim Acta 552:28
Tang Y, Lewin M, Pearce EM (2006) Macromol Rapid Commun 27:1545
Yang S, Zhang QX, HuYF (2016) Polym Degrad Stab 133:358
Qiu SL, Xing WY, Feng XM, Yu B, Mu MW, Yuen RKK, Hu Y (2017) Chem Eng J 309:802–814
Qian XD, Song L, Hu Y, Yuen RKK (2012) J Polym Res 19:9890
Lu SY, Hamerton I (2002) Prog Polym Sci 27:1661
Qian XD, Song L, Hu Y (2013) Thermochim Acta 552:87
Zhang YC, Xu GL, Yun L (2016) Thermochim Acta 643:33
Lim KS, Bee ST, Sin LT (2016) Compos Part B Eng 84:155
Jiang SH, Gui Z, Shi YQ (2014) Polym Degrad Stab 107:1
Qian XD, Pan HF, Xing WY (2012) Ind Eng Chem Res 51:85
Wang X, Hu Y, Song L (2010) Polymer 51:2435
Price D, Pyrah K, Hull TR (2002) Polym Degrad Stab 77:227
Cinausero N, Azema N, Lopez-Cuesta JM (2011) Polym Degrad Stab 96:1445
Laachachi A, Leroy E, Cochez M (2005) Polym Degrad Stab 89:344–352
Sahoo PK, Samal R (2007) Polym Degrad Stab 92:1700
Qian XD, Song L, Jiang SH (2013) Ind Eng Chem Res 52:7307
Braun U, Schartel B, Fichera MA (2007) Polym Degrad Stab 92:1528
Acknowledgements
This work was supported by Natural Science Foundation of Hebei Province (No. E2016507032, E2016507027, B2015507044), and the Natural Science Foundation of China (No.21472241).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qian, X., Jin, J., Lu, L. et al. Preparation of poly(methyl methacrylate)/silicon particle composites and the study of the properties improvement. J Polym Res 24, 45 (2017). https://doi.org/10.1007/s10965-017-1211-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1211-x