Abstract
This study investigated the effect of blending poly(styrene-co-maleic anhydride) (SMA) on the mechanical and thermal properties of nylon-6/polyether block amide (PEBA) blend. In these blends, nylon-6 was toughened with PEBA using SMA as the compatibilizer. All the blends were prepared via direct melt compounding using a co-rotating twin screw extruder. The amount of PEBA added affected the crystallization characteristics and the relative ratio of γ and α crystalline phases of Nylon 6. The crystallization rate of Nylon 6 was also affected by the cooling rate and the amount of PEBA added. The results of mechanical tests showed that the tensile properties, flexural properties, and impact strengths of the nylon-6/PEBA were all increased when blended with 1 wt% of SMA, at both 23 and −20 °C. However, for neat nylon-6, the impact strength was not affected despite that both tensile and flexural properties were increased by the blending of SMA. The results indicated that SMA can increase the compatibility between nylon-6 and PEBA, thus expanding the usage of nylon-6/PEBA blend in low-temperature applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nylon is a thermoplastics with excellent mechanical properties, and is widely employed as engineering plastics. However, its impact strength is low at temperature below 0 °C. This limits the application of nylon in low temperature climate. Improving the impact strength below 0 °C has become an important issue in the modification of nylon. In particular, the toughening of nylon has been studied extensively in the literature [1–26].
In general, the toughening is achieved by melt-blending the matrix with an elastomer possessing Tg far below room temperature. In the literature, the polymers chosen for toughening nylon included styrene-(ethylene-co-butylene)-styrene triblock copolymer (SEBS) [2, 4, 9, 14], polyethylene-octene elastomer (POE) [5, 11, 13, 16, 18, 21, 22], ethylene-propylene rubber (EPR) [2, 4, 12], ethylene-propylene diene copolymer (EPDM) [1, 3, 4, 6, 8, 17], ethylene-vinyl acetate copolymer (EVA) [7, 10, 15, 18, 21], natural rubber (NR) [26], fluoroelastomer [25], polyethylene (PE) [23], and polypropylene (PP) [2, 10, 17, 24]. These polymers are all non-polar, making them incompatible with polar nylon. In order to improve the compatibility to improve the toughening effect, compatibilizing modification was extensively investigated.
Poly(styrene-co-maleic anhydride) (SMA) has been used to improve the compatibility between nylon and other polymers [27, 28]. Toughened nylon has wide range of application including bumper, fender, snow boots, snow shoes, roller skates, fitness equipment, baby car, bicycle accessories, wheels, and office furniture.
The mechanical properties of Nylon 6, such as tensile strength, hardness, low temperature brittleness, notch sensitivity, etc., are affected by the crystalline characteristics. As a result, the methods of blending Nylon 6 with high molecular weight polymers were investigated to improve the crystalline properties [29–38]. These polymers included cyclodextrin, starch, polystyrene (PS), Nylon 66, ABS, poly(vinylidene fluoride) (PVDF), poly(propyl oxide) (PPO), cellulose, acrylate, PP, PE, styrene-butadiene rubber (SBR), acrylate rubber, EPDM, polyethylene-propylene rubber, etc. [24, 29, 39–50]
Poly(ether-block-amide) (PEBA) (Scheme 1) is a thermoplastic elastomer made from flexible polyether and rigid polyamide 11 based on renewable resources [51–54]. In this study, PEBA was employed as the toughening agent for Nylon 6, and SMA (Scheme 2) was used as the compatibilizer. The resultant nylon blend was subject to measurements of mechanical properties, impact strength, and crystallization behaviors. Based on these results, the effect of blending with toughening agent and compatibilizer on the mechanical properties of Nylon 6 was evaluated.
Experimental
Materials
Table 1 summarizes the properties of the materials used in this study. Neat Nylon 6 (Zisamide TP4208, ZigSheng, Taiwan) has a relative viscosity (RV) of 2.4 (¯M w = 20.5 kD with PDI 1.38) and the density of amino end-groups was 44 μeq g−1. Polyether block amide (PEBA) (Pebax® Rnew 40R53 SP 01) was purchased from Arkema, France. The compatibilizer, SMA, was purchased from Sigma-Aldrich, USA, with a mole ratio of styrene to maleic anhydride of 1.3 to 1.
Blending
Table 2 lists the formulation of the blends. Before processing, all materials were dried for 12 h at 80 °C in a vacuum oven to ensure removal of water. Blends were prepared in a co-rotating twin screw extruder (L/D = 42, L = 1.05 m) (ZE 25A × 42D, Thermo-Haake, Germany) operating at a screw speed of 250 rpm and a temperature profile of 205, 220, 225, 230, 235, 240, 245, 250, and 255 °C (die). Table 3 lists the details of the processing conditions.
DSC analyses
A Perkin-Elmer DSC-7 was used to measure the endotherms and exotherms of different specimens under the nitrogen environment at a nitrogen flow rate of 40 ml/min. The dynamic thermograms were obtained from specimens at a heating rate of 10 °C/min from −40 °C to 230 °C (1st heating scan), held at 230 °C for 5 min to erase the thermal history, subsequently cooled to −40 °C at a cooling rates of either 5 or 10 °C/min, and then reheated to 230 °C at a heating rate of 10 °C/min (2nd heating scan). At least three specimens prepared from hermetic aluminum (Al) pans were tested for each DSC measurement. The thermograms obtained were calibrated by the baseline obtained from the empty Al pan and the indium standard.
The crystallinity of Nylon 6 blends is calculated as follows,
where Xc is the crystallinity, ΔHm is the experimental heat of fusion at melting point determined by DSC, ΔHref is the theoretical heat of fusion of 100% fully crystalline Nylon 6 (241 J/g) [31] and w is the weight fraction of Nylon 6 in blends.
X-ray diffraction (XRD)
The hot pressed samples (pressed at 230 °C and cooled at the room temperature with a thickness about 120 μm) were conditioned in the oven at 80 °C (above the glass transition temperature of Nylon 6) for 24 h before XRD analyses. The diffraction patterns of the various samples were obtained using Cu (Kα) X-rays in a Bruker d2 phaser at a sanning rate of 0.04°/min.
Mechanical properties
The extruded pellets were injection molded into standard 0.318 cm thick Izod (ASTM D256) and tensile (ASTM D638 with type I) bars using an injection molding machine (VS-100 M, Victor Taichung Machinery Works Co., Taiwan). In addition, the flexural specimen bars were prepared as specified by ASTM D790. The barrel temperature was set up at 255 °C (nozzle) and the molding temperature at 80 °C. An injection pressure of 8 MPa and a holding pressure of 3.5 MPa were applied. The details of the processing conditions were described in Table 3.
Notched Izod impact strength was determined using an impact tester (Cs137–25, Atlas Co., Germany). The tensile testing was performed using an Instron tester (type 5567) at a crosshead speed of 50 mm/min for strength measurements and 5 mm/min for modulus measurements. In addition, flexural testing was performed also using an Instron tester (type 5567) at a crosshead speed of 14 mm/min for strength measurements and 1.4 mm/min for modulus measurements.
All specimens were kept in a sealed desiccator under vacuum for 24 h before measuring mechanical properties. The values reported were an average from five measurements.
Results and discussion
Crystallization characteristics from DSC
In general, the molecules in the crystalline and amorphous regions of Nylon 6 all can form strong hydrogen bonds because of their highly polar chemical structures. These strong hydrogen bonds restrict the intermolecular movements and regular alignment of chains, consequently, limit greatly the extent of crystallinity in Nylon 6. Nylon 6 usually has crystallinity near 30% and exhibits polymorphic structures with α and γ crystalline phases. The monoclinic α phase with intra-sheet hydrogen bonds is more stable and has a higher modulus than the metastable hexagonal γ and pseudohexagonal γ* phases with inter-sheet hydrogen bonds. Consequently, the structural differences between the α and γ phases could affect the physical and mechanical properties of Nylon 6. [34–36]
The processing conditions significantly affect the crystalline structures formed in Nylon 6. It was found that the relatively faster and slower crystallization rate would facilitate the formation of γ and α phases, respectively. A higher crystallization temperature also enhances the growth of α phase but a lower crystallization temperature enhances the growth of γ phase. The α phase is usually observed in solvent casting and annealed specimens while the γ phase is usually obtained in melt spinning and nanocomposite samples. These crystalline phases are interchangeable. The α phase can transform into γ and γ* phases or the high temperature monoclinic structure when heated. The γ phase can also change to α phase through a melting-recrystallization process when heated using a slow heating rate. The simple Nylon polymers contain mostly crystalline γ phase. [29, 30, 34–36]
Factors including heating condition, stress, molecular weight, additive, and humidity can all affect the crystallization of Nylon 6 in various degrees. Among these factors, the molecular weight significantly changes the crystallization characteristics of Nylon 6. Both low and high molecular weight Nylon 6 would form γ phase crystals at a low crystallization temperature, undergo γ → α phase transition at a middle temperature as heated, and then change to α phase at a high temperature. However, the melt of the low molecular weight Nylon 6 follows three processes while that of the high molecular weight Nylon 6 goes through five processes and has a transition zone during the melt. [30, 31, 38]
The effect of PEBA on the crystallization behavior of Nylon 6 can be observed from the cooling exotherms in Fig. 1. Table 4 summarizes the crystallization characteristics of these samples. The sample without PEBA (STN-1) exhibited higher onset temperature (Tonset = 188.2 °C) and crystallization temperature (Tc = 184.7 °C) and lower crystallization half-time (t1/2 = 0.34 min) than those samples with PEBA (STN-2 ~ 5), indicating that the presence of PEBA hindered the crystallization of Nylon 6. The reason is that the flexible PEBA has much lower crystallization temperatures (Tonset = 127.4 °C and Tc = 116.4 °C), and much smaller crystallization enthalpy (ΔHc = 25.5 J/g). Thus the addition of PEBA adversely affected the crystallization of Nylon 6 during the cooling process.
Table 4 also lists the crystallinity of the Nylon 6 constituent in these samples. The theoretical heat of fusion of 100% crystalline Nylon 6, 241 J/g, was used to calculate the crystallinity [31, 55]. Various values (230 ± 20 J/g) other than 241 J/g have been proposed for the fully crystallized α crystals of Nylon 6 [56–59] depending on the source of Nylon 6. The value used here was good for comparison purpose. The crystallinities of all the samples varied little around 27 to 28%, indicating that the crystallinity of Nylon 6 was little affected by PEBA. As a result, regardless of the presence of different amounts of PEBA, the constituent of Nylon 6 in the samples can crystallize well and attain a high crystallinity near the highest possible crystallinity of 30% for Nylon 6 [31].
Figure 2 shows the 2nd heating curves at a heating rate of 10 °C/min. Table 5 summaries the melting characteristics of Nylon 6 in these samples. The melting endotherms were affected by the addition of PEBA. In general, all the samples exhibited three clear melting peaks at temperatures above 180 °C. The smaller peaks near 183 ~ 189 °C (Tm1) are due to the small crystallites melted at the relatively lower temperature. The melting peaks at 210 ~ 212 °C (Tm2) are due to the melting of γ crystals, and the largest peaks near 219 ~ 220 °C (Tm3) are resulted from the melting of α crystals. These 3 melting temperatures were not much affected by the addition of PEBA. This result is consistent with the previous results that the crystallinity of Nylon 6 is little affected by PEBA.
Table 5 also shows that ΔHm of Nylon 6 was not much affected by the addition of PEBA. However, Fig. 2 shows that the relative intensities of three melting peaks varied with the content of PEBA. The samples STN-2 ~ STN-5 exhibited higher contents of α crystals than STN-1. Higher PEBA content led to less γ phase and more α phase. This is due to the different crystallization rate and crystallization temperature experienced among samples because of which affected the formation of α and γ crystals. Lower PEBA content could result in a higher crystallization rate and thus facilitate the formation of γ phase. This is consistent with the Tonset and Tc obtained previously. Furthermore, higher content of PEBA elastomer also resulted in a decrease of the glass transition temperature (Tg) of Nylon 6 samples confirmed by the lower Tg’s of STN-4 and STN-5 than those of STN-1 ~ STN-3 (see Table 5).
In Table 5 also shows the melting enthalpies (ΔHm) of the samples. Comparing to ΔHc listed in Table 4, all ΔHm were greater than ΔHc due to the cold crystallization and secondary crystallization happening during the 2nd heating process. Regardless of the sample type, the difference between ΔHm and ΔHc is limited because the crystallinity obtained is already high in the previous cooling process.
The crystallization behaviors were also measured by using cooling rates of 5 °C/min to investigate the effect of cooling rate on the crystallization characteristics of Nylon 6 (see Fig. 3). These characteristics were summarized in Table 6. As expected, Tonset and Tc increased at lower cooling rate for all the samples due to the kinetic effect on the crystallization process. In addition, the lower cooling rate also resulted in a longer crystallization time and a higher crystallinity of Nylon 6 than at a faster cooling condition by comparing results shown in Tables 4 and 6. It is reasonable to see in Fig. 4 that the larger melting endotherms at Tm2 due to the melting of γ crystals than those in Fig. 2 are obtained because of the different crystallization rates.
Figure 5 shows the XRD patterns taken from samples after annealing at 80 °C. All five samples exhibited small shoulders near 21.3° and large main peaks near 23.6° which were from (001) plane of γ phase and (002)/(202) planes of α phase, respectively. The result indicated that a relatively smaller amount of γ phase was in these samples comparing to the DSC samples due to the use of different cooling (crystallization) and 2nd heating processes.
Mechanical properties
Table 7 summarizes the mechanical properties of Nylon 6/SMA/PEBA blends, including impact properties, tensile properties and flexural properties.
Impact test
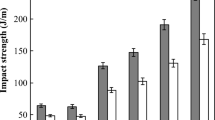
Figure 6 shows the results of impact test of Nylon 6 and its blends. The impact strength of neat Nylon 6 (STN-1-A) was 64.3 ± 2.8 J/m at 23 °C while that of Nylon 6/1 wt% SMA (STN-1) was 62.5 ± 3.1 J/m at 23 °C. The difference was insignificant. Similarly, the impact strengths at −20 °C were 48.3 ± 2.1 J/m and 47.6 ± 2.3 J/m for STN-1-A and STN-1, respectively. This indicates that the addition of 1 wt% SMA did not affect the impact strength of Nylon 6. However, for the blends of Nylon 6/PEBA, the presence of 1 wt% SMA did improve greatly the impact strength, both at 23 and −20 °C. In the case of STN-2, which contained 15 wt% of PEBA, the impact strength was significantly increased by 49% and 36% at 23 and −20 °C, respectively. The increase can be attributed to the improvement in the compatibility between Nylon 6 and PEBA by the compatibilizer, SMA.
Figure 7 shows the effect on PEBA content on the impact strength of the Nylon 6/PEBA/SMA blends. The impact strength of the blend increased with the PEBA content. In particular, the blend containing 35 wt% of PEBA (STN-5) exhibited an impact strength 5.47 folds of that without PEBA (STN-1) at 23 °C, and 5 folds at −20 °C. This indicates that the presence of PEBA can greatly improve the impact strength of Nylon 6.
The improvement in the impact strength by PEBA is from the flexible polyether segment. As indicated in the literature, flexible molecular chains can increase the impact properties of Nylon 6 [5, 6, 16].
Tensile properties
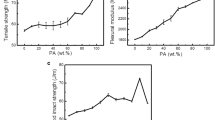
Figure 8 shows the effect of PEBA on the tensile properties of STN with 1 wt% SMA. The tensile strength decreased slightly by 9% when the PEBA content was below 15%, while that decreased drastically by 33% when PEBA content was increased to 35%. This can be attributed to the presence of lower-strength PEBA. On the other hand, the tensile modulus decreased almost linearly from 2.81 to 1.42 GPa with the increase of PEBA content. This is because PEBA diluted the crystalline Nylon 6 and making the blend softer.
Flexural tests
Figure 9 shows the effect of PEBA content on the flexural properties of Nylon 6/1 wt% SMA. The flexural strength and modulus decreased by 55% and 56%, respectively, when the PEBA content increased to 35 wt%. This is reasonable since PEBA is a soft polymer with a flexural modulus of only 75 MPa [60].
Conclusions
The mechanical properties of Nylon 6 were affected by melt-blending PEBA. In addition, by adding a compatibilizer (SMA), the compatibility between Nylon 6 and PEBA was improved, as revealed by the increase in the mechanical properties. The impact strength of the blend with 15% of PEBA was about 200% of that of neat Nylon 6 at 23 °C, and with the addition of 1% of SMA, the impact strength was about 300% of that of neat Nylon 6. Furthermore, the reduction in the impact strength at −20 °C was smaller for the blend of Nylon 6/PEBA than neat Nylon 6. This preserving in the impact strength was further increased when SMA was added. In addition, the impact strength increased with the content of PEBA and SMA.
The results of DSC indicated that the presence of PEBA would affect the crystallization rate and the relative ratio of γ and α crystalline phases formed in Nylon 6. The amount of PEBA added changed the crystallization characteristics and also the relative amounts of γ and α crystals in Nylon 6. It was confirmed that the cooling rate and the amount of PEBA added could considerably affect the crystallization rate of Nylon 6.
Unlike impact strength, the tensile strength and modulus of the blend decreased with the increase of the content of PEBA and SMA. Similarly, the flexural properties also decreased with the increase of the content of PEBA and SMA. However, the extent of reduction was around 30 ~ 50%, which was acceptable considering the improvement in the impact strength.
References
Gaymans RJ, Borggreve RJM (1988) Integr Fundam Polym Sci Technol 2:248
Gonzhlez-Montiel A, Keskkula H, Paul DR (1995) Polymer 36:4621–4637
Wang XH, Zhang HX, Jiang W, Wang ZG, Liu CH, Liang HJ, Jiang BZ (1998) Polymer 39:2697–2699
Lu M, Keskkula H, Paul DR (1995) J Appl Polym Sci 58:1175–1188
Yu ZZ, Ou YC, Qi ZN, Hu GH (1998) J Polym Sci B Polym Phys 36:1987–1994
Wang Y, Wang W, Peng F, Liu M, Zhao Q, Fu PF (2009) Polym Int 58:190–197
Yu H, Zhang Y, Ren W (2009) J Polym Sci B Polym Phys 47:877–887
Okada O, Keskkula H, Paul DR (2004) J Polym Sci B Polym Phys 42:1739–1758
Wu D, Wang X, Jin R (2006) J Appl Polym Sci 99:3336–3343
Liu H, Xie T, Hou L, Ou Y, Yang G (2006) J Appl Polym Sci 99:3300–3307
Li QF, Kim DG, Wu DZ, Lu K, Jin RG (2001) Polym Eng Sci 41:2155–2161
Choudhury A, Balmurulikrishnan A, Sarkhel G (2008) Polym Adv Technol 19:1226–1235
Yu ZZ, Ke YC, Ou YC, Hu GH (2000) J Appl Polym Sci 76:1285–1295
Xie T, Yang G (2004) J Appl Polym Sci 93:1446–1453
Yu H, Zhang Y, Ren W (2009) J Polym Sci B Polym Phys 47:434–444
Peng J, Qiao J, Zhang S, Wei G (2002) Macromol Mater Eng 287:867–870
Wang BB, Wei LX, Hu GS (2008) J Appl Polym Sci 110:1344–1350
Yu H, Zhang Y, Ren W (2009) Polym Eng Sci 49:2393–2399
Araújo EM, Jr EH, Carvalho AJF (2003) J Appl Polym Sci 90:3512–3518
Du L, Yang G (2010) Polym Eng Sci 50:1178–1185
Yu H, Zhang Y, Ren W, Hoch M, Guo S (2011) J Appl Polym Sci 121:3340–3346
Kumar S, Satapathy BK, Maiti SN (2013) Polym Adv Technol 24:511–519
Kudva RA, Keskkula H, Paul DR (1999) Polymer 40:6003–6021
Afshari M, Kotek R, Kish MH, Dast HN, Gupta BS (2002) Polymer 43:1331–1341
Banerjee SS, Bhowmick AK (2013) Polymer 54:6561–6571
Tanrattanakul V, Sungthong N, Raksa P (2008) Polym Test 27:794–800
Chiang CR, Chang FC (1997) Polymer 38(19):4807–4817
Qin SH, Yu J, Zheng Q, He M, Zhu H (2008) Chin J Polym Sci 26(1):73–80
Ren X, Wu G, Zhang X (2011) 2011 2nd international conference on chemistry and chemical engineering. IPCBEE 14:125–129
Liu T, Tjiu WC, He C, Na SS, Chung TS (2004) Polym Int 53:392–399
Fornes TD, Paul DR (2003) Polymer 44:3945–3961
Murthy NS, Aharoni SM, Szollosi AB (1985) J Polym Sci Polym Phys Ed 23:2549–2565
Zhang CF, Liu YH, et al. (2009) Sci China B:Chem 39(11):1378–1385
Dasgupta S, Hammond WB, Goddard III WA (1996) J Am Chem Soc 118:12291–12301
Liu Y, Cui L, Guan F, Gao Y, Hedin NE, Zhu L, Fong H (2007) Macromolecules 40(17):6283–6290
Katoh Y, Okamoto M (2009) Polymer 50:4718–4726
Rwei SP, Tseng YC, Chiu KC, Chang SM, Chen YM (2013) Thermochim Acta 555:37–45
Zapata-Espinosa A, Medellín-Rodríguez FJ, Stribeck N, Almendarez-Camarillo A, Vega-Díaz S, Hsiao BS, Chu B (2005) Macromolecules 38:4246–4253
Miyata K, Watanabe Y, Itaya T, Tanigaki T, Inoue K (1996) Macromolecules 29:3694–3700
Vo LT, Giannelis EP (2007) Macromolecules 40:8271–8276
Tedesco A, Barbosa RV, Nachtigall SMB, Mauler RS (2002) Polym Test 21:11–15
Kim KJ, Cho HW, Yoon KJ (2003) Eur Polym J 39:1249–1265
Khatua BB, Lee DJ, Kim HY, Kim JK (2004) Macromolecules 37:2454–2459
Oderkerk J, Groeninckx G (2002) Macromolecules 35:3946–3954
Beltrán Á, Gómez-Emeterio BP, Marco C, Ellis G, Parellada MD, Díaz-Requejo MM, Corona-Galván S, Pérez PJ (2012) Macromolecules 45:9267–9274
Biber E, Gunduz G, Mavis B, Colak U (2010) Mater Chem Phys 122:93–101
Lai SM, Li HC, Liao YC (2007) Eur Polym J 43:1660–1671
Wu D, Wang X, Jin R (2004) Eur Polym J 40:1223–1232
Huang JW, Chang CC, Kang CC, Yeh MY (2008) Thermochim Acta 468:66–74
Psarski M, Pracella M, Galeski A (2000) Polymer 41:4923–4932
Kim JH, Ha SY, Lee YM (2001) J Membr Sci 190:179–193
Sjong A, Villagomez F, Kendale A (2006) ANTEC plastics. In: Annual technical conference proceedings. Society of Plastic Engineers, Charlotte, pp. 1544–1548
Murray KA, Kennedy JE, McEvoy B, Vrain O, Ryan D, Cowman R, Higginbotham CL (2013) J Mech Behav Biomed Mater 17:253
Todros S, Natali AN, Piga M, Giffin GA, Pace G, Noto VD (2013) Polym Degrad Stab 98:1126–1137
Illers KH (1978) Makromol Chem 179(2):497–507
Khanna YP, Kuhn WP (1997) J Polym Sci B Polym Phys 35:2219
Wunderlich B (1980) Macromolecular physics. Crystal melting. Vol. 3. Academic Press, New York
Gogolewski S, Gasiorek M, Czerniawska K, Pennings AJ (1982) Colloid Polym Sci 260(9):859–863
Starkweather Jr HW, Zoller P, Jones GA (1984) J Polym Sci, Polym Phys Ed 22(9):1615–1621
The technical datasheets of Pebax® Rnew 40R53 SP 01, Retrieved from http://www.pebax.com/export/sites/pebax/.content/medias/downloads/literature/tds-pebaxrnew-40r53sp01.pdf
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, WM., Yang, MC., Hong, SG. et al. Effect of poly(styrene-co-maleic anhydride) on physical properties and crystalline behavior of nylon-6/PEBA blends. J Polym Res 24, 40 (2017). https://doi.org/10.1007/s10965-017-1201-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1201-z